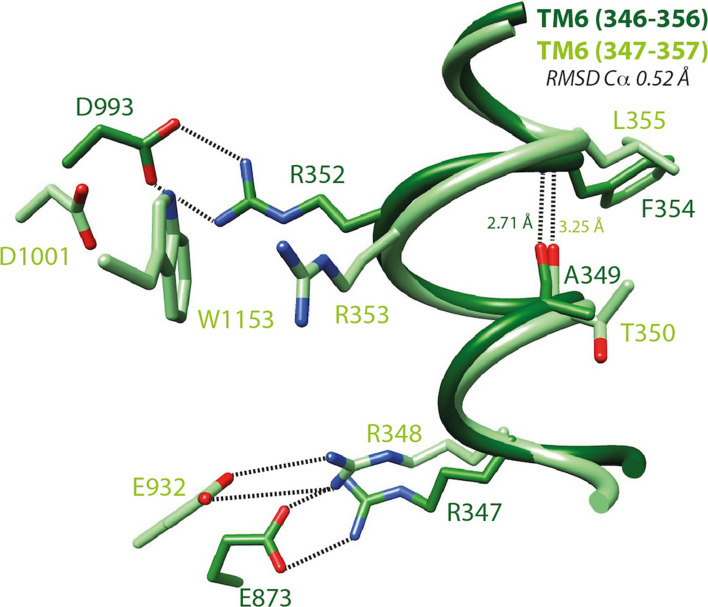
Fig. 4.
S-closed/open forms—focus on TM6. Local superimposition of the TM6 segment including a π-bulge within the s-closed form (cryo-EM, zebrafish CFTR, PDBID:5W81 [8], light green) and the open form (model, human CFTR, this study, dark green) of CFTR. The depicted amino acids are equivalent between human and zebrafish CFTR, expect from E873 (human) and E932 (zebrafish), corresponding to E871 (zebrafish) and D924 (human), respectively. An H-bond between i (human A349/zebrafish T350) and i + 5 (human F354/zebrafish L355) stabilizes this local conformation, which is located in close vicinity of the two basic residues involved in salt bridges typical of the open (human R352/zebrafish R353; salt bridge with human D993/zebrafish D1001) and closed (human R347/zebrafish R348; salt bridge with human D924/zebrafish E932) forms, respectively. Of note are (1) the impossibility of forming directly a salt bridge typical of the open form in the reported s-closed conformation due to the presence of an intercalating tryptophane (zebrafish W1153, human W1145) and (2) the presence in the open form (model) of an alternate bond involving R347 and E873. Within this segment, the putative accessibility of V350 (V351 in zebrafish CFTR, not shown), discussed in a previous article [14], can be well understood regarding these data: V350 is totally buried in the open state of the channel and partially exposed to the solvent in the apo and s-closed states. V350 is also one of the four pivots described in Supplementary Data 16