Fig. 9.
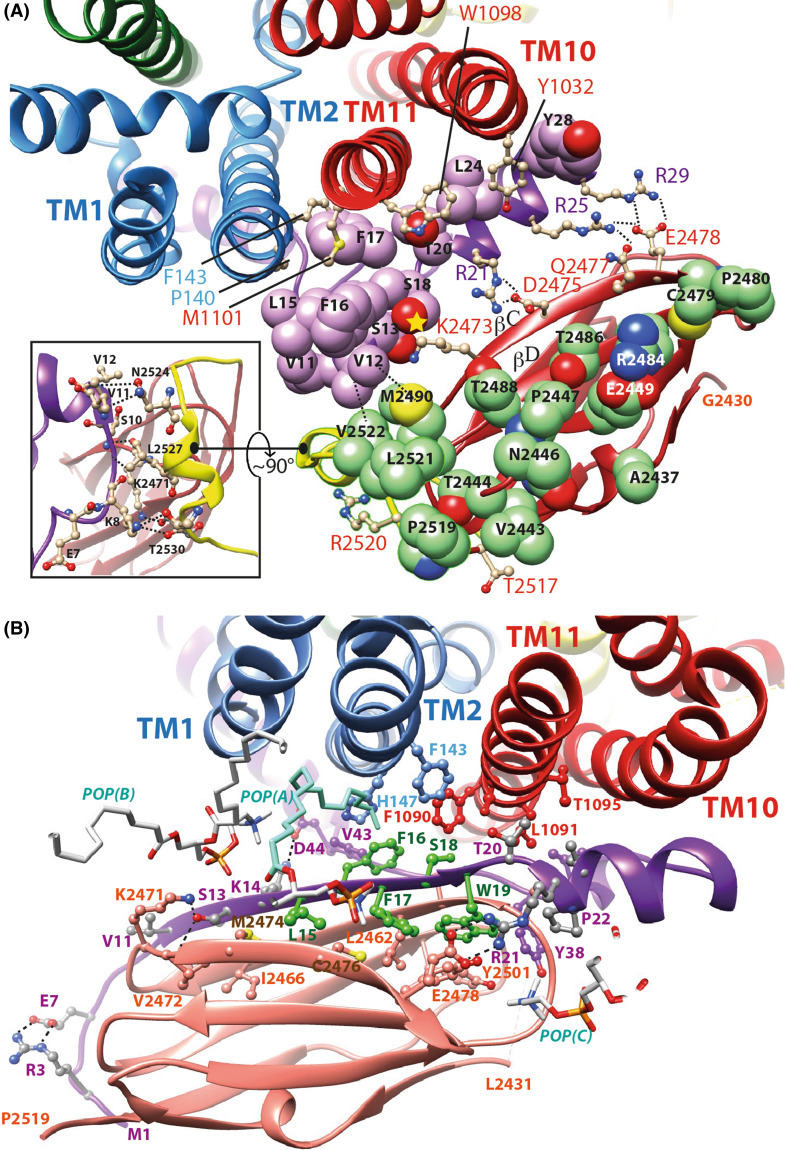
Two possible models of the hypothetical association of the human filamin IG23 domain with CFTR N-terminus lasso. a Model of the FlnA IG23/CFTR Lh1 complex, which repeatedly showed a good stability after 50 ns of MD simulations. The carbon atoms of the CFTR Lh1 lasso are colored in purple, those of FlnA IG23 in green. Two main interaction regions are present in this model: at right polar contacts with Lh1 S13, S18, R21, R25, and R29 and at left hydrophobic contacts involving Lh1 V11, V12, V15, and F16 with IG23 V2522, M2490, and L2521. Amino acids of TM2, TM10, and TM11 interacting with the lasso Lh1 are depicted in ball and sticks. F143, P140, M1101, W1098, and Y1032 are at the same level with respect to the membrane than the IG23 amino acids depicted in green. In the inset are indicated the contacts between the lasso segment including aa K8–V11 and the beginning of the IG23–IG24 linker (in yellow), which may strengthen the stability of the complex. This region lies nearly perpendicular to the Lh1/IG23 main contact shown in this panel. b Model of the FlnA IG23/CFTR modified Lh1 (aa 1–21). Amino acids 1–21 are here considered in a different, extended conformation (β-strand between V11 and T20), all the other parts of the lasso remaining unmodified. The conformation presented here is stable after 50 ns MD simulation and displays favorable interactions with the TM1, TM2, and TM11 helices as well as with the membrane lipids. Three of them are depicted [POP(A), POP(B) and POP(C)]. The POP(A) chain colored in light blue (between TM2 and CFTR F16) stayed very stable along the MD simulation. The sequence L15–W19 is colored in green. Of note is that the zebrafish CFTR sequence (Y15–F16–F17–W18–W19), like several ABC exporters, possesses an aromatic amino acid at position 18. The L15Y and S18W substitutions are fully compatible with the present model, with the following immediate contacts [CFTR Y15 = FlnA M2474 and C2476, CFTR F17, POP(A), CFTR F16 = POP(A), CFTR V43, H147 and F1090, CFTR F17 = FlnA C2476 and Y2483, CFTR Y15 and W19, CFTR W18 = FlnA 2461, CFTR W19, F143, F1090, L1091, CFTR W19 = FlnA T2454, L2462 and Y2483, CFTR F17, and W18]. The orientation of FlnA IG23 domains is globally similar for the two models, with a relatively small translation toward TM1 and TM2 (~ 8 to 9 Å). IG23 E2478 establishes a salt bridge with R25/R29 in the first model (a) and with R21 in the second one (b)