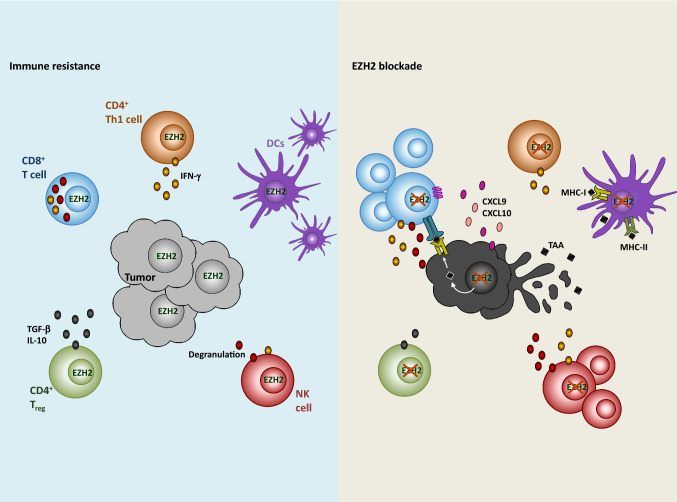
Fig. 3.
EZH2-mediated immune resistance in anti-tumor immune responses. EZH2 expression (left) mediates downregulation of T-cell-attracting chemokines resulting in decreased T-cell infiltration to the tumor site. The lack of antigen presentation limits T-cell effector functions. Upon EZH2 blockade (right), expression of CXCL9 and CXCL10 increases and promotes CD8+ T-cell (blue) infiltration. The recognition of cancer cells by CD8+ T cell through the MHC-I–TAA–TCR complex leads to the secretion of effector molecules, such as perforin and granzymes (dark red). CD4+ T cells (orange) require EZH2 for their differentiation and maintenance. Thus, the function of Th1 cells and regulatory T (Treg) cells can be altered upon EZH2 blockade, including production of IFN-γ, interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). EZH2 is important for infiltration of dendritic cells (DCs; purple) to the inflammatory microenvironment. However, EZH2-dependent downregulation of TAA affects DC-mediated priming of T-cells. Natural killer (NK) cells (red) mature and show enhanced lytic activity upon EZH2 inhibition. In summary, although EZH2 inhibition can affect DC migration and Th1 cell function, different mechanisms involved in tumor resistance can be efficiently reversed, thus improving tumor control