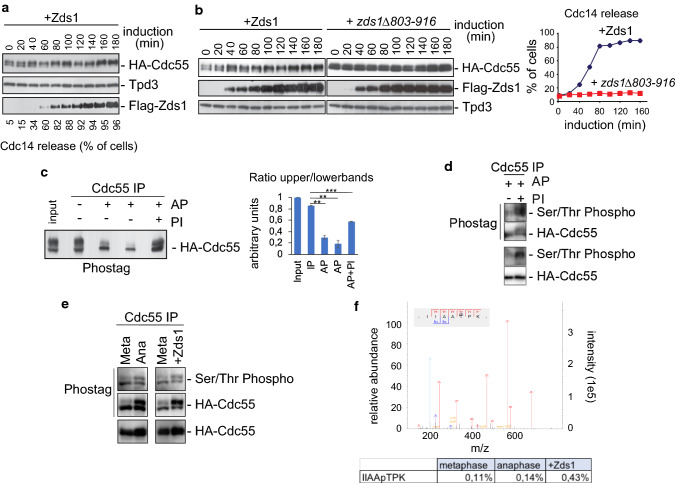
Fig. 1.
PP2A regulatory subunit Cdc55 is phosphorylated upon PP2ACdc55 downregulation. a PP2ACdc55 downregulation induces the accumulation of a Cdc55 post-translational modification. Strain Y888 was arrested in metaphase by Cdc20 depletion, and Zds1 ectopic expression was induced by galactose addition. Cdc55, Tpd3 and Zds1 proteins were analyzed by western blot. Cdc14 release from the nucleolus was visualized by immunofluorescence. b Ectopic expression of a non-functional version of Zds1 cannot promote the Cdc55 post-translational modification. Strains Y769 and Y770 were arrested in metaphase by Cdc20 depletion, and Zds1 ectopic expression was induced. Cdc55, Zds1 and Tpd3 proteins were analyzed by western blot (left). Tpd3 was used as a loading control. Cdc14 release from the nucleolus was visualized by immunofluorescence (right). c Cdc55 experiences phosphorylation modifications. Strain Y1015 was arrested in metaphase by Cdc20 depletion, and Zds1 ectopic expression was induced. After 180 min of Zds1 induction, protein extracts were prepared, Cdc55 was immunoprecipitated, and alkaline phosphatase (AP) treatment was performed. 2x PhosSTOP (Roche) was used as an alkaline phosphatase inhibitor (PI). A representative experiment of one of the assays is shown (left). A quantification of the slower and faster Cdc55 migration isoforms is depicted (right). Mean values and SEM are shown. d Cdc55 is phosphorylated in Ser/Thr residues. Cdc55 immunopurified as in c were detected using an anti-phosphoserine/threonine antibody and anti-HA as a control. One independent experiment is shown. e Phosphorylation of Cdc55 in Ser/Thr residues is enhanced in anaphase and upon Zds1 induction. Protein extracts from strain Y2541 arrested in metaphase (Meta) by Cdc20 depletion, and 20 min (Ana) after released into synchronous anaphase were prepared. Cell extract from Y1015 after 180 min of Zds1 induction was also prepared. Cdc55 was immunopurified, resolved in phostag gels and Ser/Thr phosphorylation was detected using anti-phosphoserine/threonine antibody. Purified HA–Cdc55 protein was also detected by western blot in phostag and normal gels as controls. f In vivo phosphorylation of Cdc55 at T174. Cdc55 immunopurified as in e were submitted to mass-spectrometry. The best MS/MS spectrum corresponding to the anaphase sample is shown. The ratios of modified/unmodified peptide are depicted