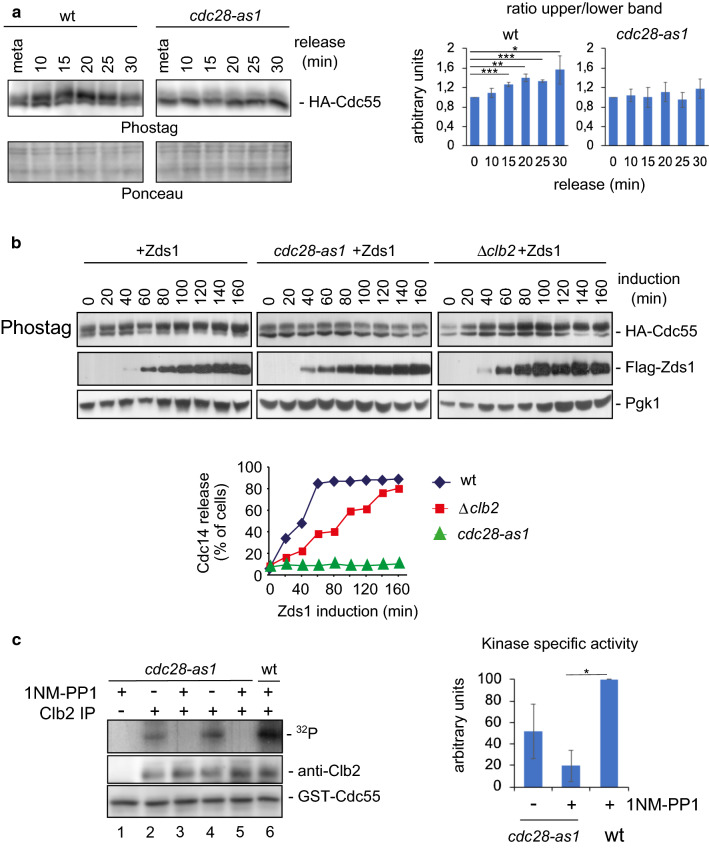
Fig. 2.
Cdk1–Clb2 complex phosphorylates Cdc55. a Cdk1 is required for Cdc55 phosphorylation. Strains Y2541 and Y492 were arrested in metaphase by depletion of Cdc20, the cdc28-as1 allele was inactivated by addition of 1 μM 1NM-PP1 20 min before release into anaphase by Cdc20 re-addition. Cdc55 protein phosphorylation was analyzed by western blot. Ponceau staining is presented as loading control. A representative experiment of one of the assays is shown (left). Quantifications of the slower and faster Cdc55 migration isoforms are depicted (right). Mean values and SEM are illustrated. b The Cdk1–Clb2 complex is necessary for proper Zds1-induced Cdc55 phosphorylation. Strains Y1015, Y537, and Y1125 were arrested in metaphase, the cdc28-as1 allele was inhibited by the addition of 1NM-PP1 during 20 min, and Zds1 expression was induced. Cdc14 release from the nucleolus was monitored by in situ immunofluorescence (bottom) and the Cdc55 phosphorylation and Zds1 levels were analyzed by western blot. Pgk1 was used as loading control. c The Cdk1–Clb2 complex phosphorylates Cdc55 in vitro. Strains Y564 and Y480 were arrested in metaphase by Cdc20 depletion and the cdc28-as1 allele was inactivated using 1 μM 1NM-PP1. Clb2 was purified by immunoprecipitation, and the Cdc28–Clb2 kinase assay was performed using bacteria-purified GST-Cdc55(1–193) as the substrate. An immunoprecipitation using rabbit IgG, instead of the Clb2 antibody, was used as a negative control for the kinase assay. A representative image of one of the experiments is depicted (left). A quantification of four independent immunoprecipitations was performed (right). Mean values and SEM are shown