Fig. 4.
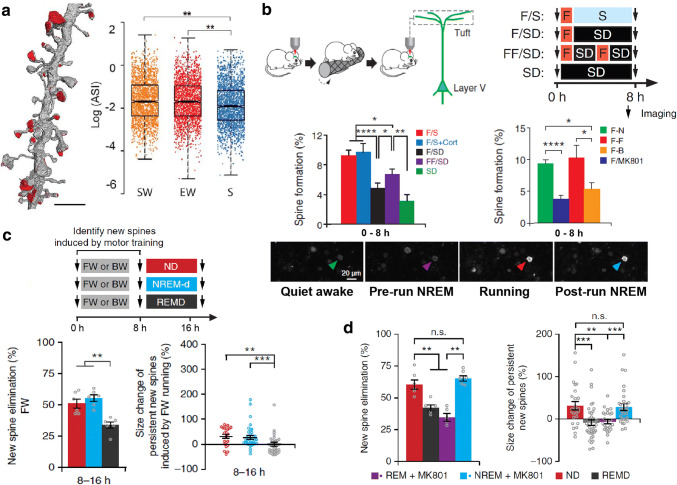
Sleep and cortical plasticity. a Distribution of axon–spine interface (ASI) sizes in layer II of primary motor (M1) and primary somatosensory (S1) cortices of mice in 3 different conditions: killed after SW, spontaneous wake at night; EW, enforced wake during the day through the exposure to novel objects or S, ad libitum sleep during the day. Results display a significant net reduction in ASI sizes after sleep (S) compared to the other groups [202]. b Top left: transcranial two-photon imaging to track motor cortex layer V pyramidal neuron apical dendritic spines from head-restrained wake transgenic mice before and after running in a rotarod paradigm. Top right and middle left: sleep deprivation during ~ 7 h immediately after motor training significantly attenuates the training-induced motor cortex spine formation. F forward running, SD sleep deprivation, S sleep, Cort. corticosterone injection. Bottom: two-photon calcium imaging of animals expressing GCaMP6 reveals a NREM sleep-related reactivation of cells activated during motor training. Middle right: injection of the NMDAr blocker MK801, as well as a different learning (B backward running) reduces the rate of training-induced spine formation in the motor cortex (modified from [208]). c Using the same two-photon imaging protocol of head-restrained mice as in b, the authors reported that the majority of the new spines formed in the motor cortex during the first 8 h after motor training are eliminated within the next 8 h, as opposed to the majority (~ 70%) of the persistent new spines which increase in size. Both processes were impaired by selective REM sleep deprivation within this late 8 h-time window. d Selective MK801 pulse injection into the motor cortex at the beginning of REM sleep episodes impaired the elimination of training-induced new spines and the size increment of persistent new spines.
(c and d modified from [ 114 ])