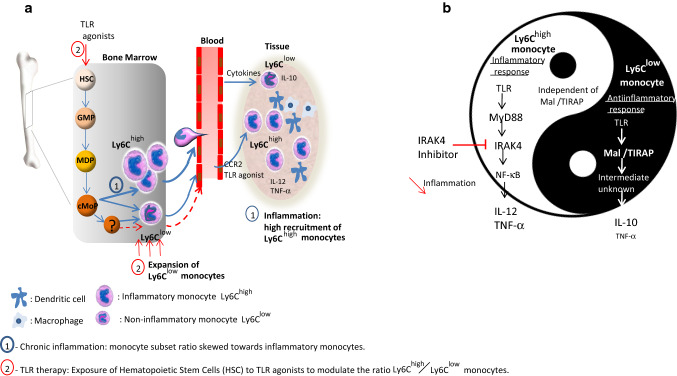
Fig. 2.
Different therapeutic strategies to control the inflammatory activity of monocytes based on a mouse model. During infection and chronic inflammation, Ly6Chigh monocytes that derived from granulocyte-monocyte progenitor (GMPs), monocyte-dendritic cell progenitor (MDP) and common monocyte progenitor (cMoP), egress from the bone marrow and selectively traffic to sites of inflammation. The Ly6Chigh monocyte egression requires expression of the chemokine receptor CCR2. The migration of Ly6Chigh monocyte to tissue where they can retain their own properties without differentiating into macrophage or dendritic cell is prominent and robust. Ly6Chigh monocytes can theoretically convert into Ly6Clow cells although this is debatable due to the possible existence of Ly6Clow progenitors not yet identified [68]. When the antiinflammatory activity of Ly6Clow monocytes is not able to counteract the inflammatory mediator release, the inflammatory state persists causing tissue damage. In this context, a therapeutic intervention is necessary to return to homeostasis. a One strategy may consist in the manipulation of the hematopoietic stem cell (HSC) development by systemic administration of TLR agonist (alone or in association) that may change the ratio Ly6Chigh versus Ly6Clow monocyte number by promoting the expansion, egress, and activation of Ly6Clow monocytes. b The second strategy is based on the differential use of signalling pathways by monocyte subpopulations according to Gravina et al. [41] allowing to specifically target inflammatory monocytes. In a context where proinflammatory cytokines are released by Ly6Chigh monocytes in a MyD88/IRAK4-dependent way and antiinflammatory cytokines are produced by Ly6Clow monocyte in a Mal/TIRAP- dependent way, IRAK4 inhibitors appear relevant to treat exacerbated inflammatory disorders. This may allow to specifically reduce inflammatory mediators released by Ly6Chigh monocytes without altering repair functions of other monocyte populations