Abstract
Following the serendipitous discovery of the ageing suppressor, αKlotho (αKl), several decades ago, a growing body of evidence has defined a pivotal role for its various forms in multiple aspects of vertebrate physiology and pathology. The transmembrane form of αKl serves as a co-receptor for the osteocyte-derived mineral regulator, fibroblast growth factor (FGF)23, principally in the renal tubules. However, compelling data also suggest that circulating soluble forms of αKl, derived from the same source, may have independent homeostatic functions either as a hormone, glycan-cleaving enzyme or lectin. Chronic kidney disease (CKD) is of particular interest as disruption of the FGF23–αKl axis is an early and common feature of disease manifesting in markedly deficient αKl expression, but FGF23 excess. Here we critically discuss recent findings in αKl biology that conflict with the view that soluble αKl has substantive functions independent of FGF23 signalling. Although the issue of whether soluble αKl can act without FGF23 has yet to be resolved, we explore the potential significance of these contrary findings in the context of CKD and highlight how this endocrine pathway represents a promising target for novel anti-ageing therapeutics.
Keywords: Fibroblast growth factor, Klotho proteins structural biology, Crystallography, Kidney disease, Cardiovascular disease, Receptors, Therapeutics, Phosphate
Introduction
From its chance discovery several decades ago [1], a growing body of evidence has revealed a pivotal role for αKlotho (αKl) in renal mineral physiology but also as a ubiquitous protection factor mitigating the effects of diverse insults in multiple tissues [2]. Central to this work has been an appreciation of how transmembrane αKl has evolved as a co-receptor for fibroblast growth factor (FGF)23 [3], an osteocyte-derived hormone that maintains mineral ion homeostasis by regulating functions in the kidney, and to a lesser extent, the parathyroid glands. Besides membrane αKl, soluble forms also exist [4, 5], which are purported to confer an impressive array of cytoprotective effects (e.g., anti-oxidant, anti-inflammatory, and anti-senescent) as well as inhibitory effects on signalling molecules (e.g., Wnt, TGF-β1, IGF-1, and insulin), all independent of canonical FGF23–FGF receptor (FGFR) signalling and in tissues that do not express membrane αKl [6–20].
αKl was discovered quite accidently during attempts to generate transgenic mice overexpressing the type I sodium-proton exchanger [21]. Random insertion of multiple copies of the transgene in the 5′ flanking region of the klotho gene resulted in virtually undetectable expression by inadvertently disrupting its promoter. Mice homozygous for this disrupted allele were characterised by a short life span, growth retardation and a number of pathological features commonly associated with old-age in humans: skin atrophy, muscle wasting, arteriosclerosis, and senile osteoporosis [1]. The striking premature ageing phenotype of kl/kl hypomorphic mice has now spawned over 2 decades of research into this enigmatic anti-ageing factor. That over-expression of αKl rescued the phenotype of these αKl-deficient animals has in more recent times led to interest in exogenous αKl-based therapeutics for age-related disorders like chronic kidney disease (CKD) [14, 22, 23].
Although recent studies have greatly advanced our understanding of the structural interactions that underpin the formation and activity of FGF23–FGFR–αKl signalling complex, controversies persist in relation to the physiological functions of soluble αKl and whether it can act directly without FGF23 [24]. Moreover, the pathways responsible for the ageing phenotypes associated with disturbances in the FGF23–αKl axis also remain contentious. The purpose of the current review is to update the reader on these developments and discuss the implications of these findings in the context of CKD and emerging therapeutic interventions.
Endocrine FGF signalling: the need for αKlotho
Phylogenetic relationships classify FGF23 as a member of the endocrine FGF19/21/23 subfamily, which derived from a canonical Fgf4-like ancestral gene following several global and local gene duplication events that occurred in early vertebrate evolution [25, 26]. Although the endocrine FGFs show minimal sequence homology, they are all implicated in homeostatic regulatory networks with effects on bile acid synthesis (FGF19), glucose/lipid metabolism (FGF21) and mineral metabolism (FGF23) [26]. Principally, FGF23 serves as a potent phosphaturic factor and counter-regulatory hormone for vitamin D hormone synthesis [27, 28]. Other newly described functions of FGF23 signalling involve sodium- and calcium-conserving effects in the distal convoluted tubules [29, 30].
Paracrine FGFs, the prototypical example being FGF2, exhibit high affinity for heparan sulphate proteoglycans (HS) which serve as obligatory co-factors for paracrine FGF signalling (Fig. 1a) [31]. HS, abundant at the cell surface and in the extracellular matrix, avidly sequester FGFs and thus limit the distance over which they can act. Consequently these FGFs signal in a paracrine or autocrine fashion, binding to the extracellular domain of one of four FGF receptor (FGFR) tyrosine kinases, encoded by Fgfr1–4 in mammals [25].
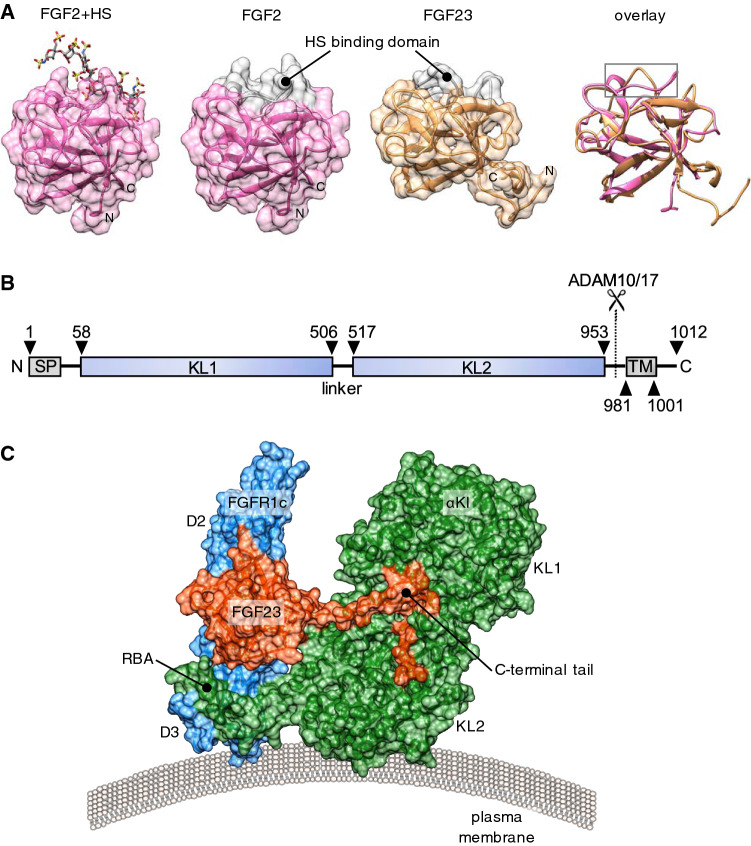
Fig. 1.
Structural basis for the endocrine action of FGF23. a From left to right: Surface representation of prototypical paracrine FGF2 with bound heparan sulphate (HS) octasaccharide (Protein Data Bank, PBD ID 1fq9) [172], aligned FGF2, FGF23 (Tyr25 to Asn170; PBD ID 2p39), and the cartoon structures overlayed [35]. Note the conserved globular core architecture but different conformations of the HS-binding (grey colour). b Cartoon depicting domain structure of 1012 amino acid human αKl. SP, TM, KL1, KL2: denotes signal peptide, transmembrane domain, tandem internal homologous KL repeats 1 and 2. The αKl ectodomain comprising KL1 and KL2 can be cleaved by plasma membrane-tethered ADAM10 or ADAM17 and released into the extracellular fluid. c Surface representation of the FGFR1c–αKl–FGF23 ternary complex from PDB ID 5w2 [48], which depicts D2 and D3 domain of human FGFR1c (blue), KL1 and KL2 domains of the ectodomain of human αKl (green) and human FGF23 (red; Tyr25 to Ser205). Note receptor binding arm (RBA) of KL2, which is necessary to stabilise the FGFR1c–αKl interaction and the tethering of the C-terminal tail of FGF23 through the KL1–KL2 cleft. Crystal structures were visualised in UCSF Chimera ver.1.13.1 [173]. Alignment was performed using the MatchMaker tool and the Needleman-Wunsch algorithm with BLOSUM-62 matrix
The ectodomain of most FGFRs comprises three immunoglobulin-like domains, D1–D3, with the two most proximal to the plasma membrane surface (D2 and D3) indispensable for FGF binding [32]. HS tethers FGFs near to the cell surface and promotes stable FGFR binding and complex homodimerisation [31]. Once bound, the receptor transphosphorylates intracellular kinase domains, activating intermediates such as FRS2α and PLCy and signal transduction through RAS–MAPK/PI3K–AKT and calcium-dependent calcineurin-NFAT pathways, respectively [33]. FGF activity is regulated by HS structure, ligand dimerisation and/or proteolysis, with specificity also imparted by alternative splicing of the c-terminal portion of D3 domain of FGFR1–3 into b and c isoforms [31].
During the divergence of endocrine FGFs like FGF23 from their paracrine forerunners, this affinity for HS was partially lost (Fig. 1a), allowing them to diffuse away from the site of production and convey signals to more distant targets via the circulation [31, 32, 34, 35]. However, endocrine FGFs could not by themselves support stable FGFR interaction at physiological levels, and so for endocrine signalling to evolve, novel receptor binding partners were needed to facilitate more stable interactions with FGFRs. Interestingly, αKl appears to have evolved to perform this co-receptor function for FGF23 around the time that vertebrates committed to a calcium phosphate-based endoskeleton [36].
FGF23:FGFR signalling in the presence of αKlotho
The Kl gene comprises 5 exons which in humans encodes a 1012 amino acid single-pass transmembrane protein, comprising a very short intracellular chain (9aa)—with no known functional domain—a transmembrane region (20aa), and a more extensive ectodomain consisting of two tandem homologous β-glycosidase-like domains: KL1 and KL2 (Fig. 1b) [1]. The Kl gene is highly conserved in mammals with syntenic loci in humans (chr 13), mice (chr 5) and rats (chr 12) [4]. Expectedly, protein sequences are also highly homologous across species with human and mouse αKl displaying 98% identity. Orthologous genes have also been identified in C. elegans and D. rerio although the transcribed product has yet to be characterised.
Regulatory gene elements also appear well conserved between species, with approximately 70% sequence identity between human and mouse promoters. Since multiple Sp1 consensus binding sites are present in the proximal promoter region, Sp1 transcription factors are predicted to be major positive regulators of Kl [37, 38]. More distal regions from the initiation start site (− 300 bp), containing consensus sequences for E-box and Ap2, are thought to be principally inhibitory [37]. In terms of physiological regulation, the Kl promoter contains multiple functional vitamin D response elements (VDRE) and a non-canonical peroxisome proliferator-activated receptors (PPAR)-response element in the 5′-flanking region (3686–3698 bp upstream of ATG) [39, 40]. The latter confers transcriptional responsiveness to thiazolidinediones, which are ligands for PPARγ. Both aldosterone and angiotensin II suppress transcription, whereas RAAS blockade enhances renal Kl expression [41, 42], independent of effects on blood pressure and proteinuria, but the underlying mechanism of these actions are unclear.
FGF23 may also be a negative regulator of αKl itself [43], firstly by indirect effects of suppression of vitamin D activation, but also directly, although the mechanism for this direct action remains elusive. Consequently, some of the pathways identified as being negative regulators of αKl (e.g., inflammatory [44], hypoxia [45]), may act by stimulating FGF23 synthesis and down-regulating αKl indirectly.
Transmembrane αKl constitutively associates only with the c-isoform of membrane FGFR1 and FGFR3 (but not FGFR2) as well as with FGFR4 on co-expressing cells [3, 46, 47]. Hence tissue-specific differences in FGFR isoform expression imparts a degree of FGF23:αKl signalling specificity. The recently solved crystal structure of the FGFR1c:αKl ectodomain:FGF23 ternary complex reveals that this interaction generates a new high-affinity composite binding groove for FGF23 [48], distinct from the binding pocket used by paracrine FGF, at the interface of the D2/D3 domains of FGFR1c and a cleft between KL1 and KL2 of αKl (Fig. 1c). Critically, αKl functions as a scaffold for the complex, simultaneously tethering FGF23 by its C-terminus and the D3 domain of FGFR1c via a receptor binding arm (RBA) which extends from KL2 [48]. Although FGF23 has a much reduced affinity for HS compared to paracrine FGFs and this property appears dispensable for FGFR binding, it does nonetheless remain essential for dimerization of the ternary complex to form a symmetric 2:2:2:2 quaternary unit and subsequent receptor activation [48]. Whether other FGFR isoforms participate in analogous scaffolds with αKl remains to be determined. Theoretically, the formation of binary FGFR:αKl complexes may also preclude these FGFRs from participating in paracrine signalling [47, 49]. Importantly, while αKl enhances the binding affinity of FGF23 for FGFR1c more than 20-fold [49], this remains an order of magnitude less than the affinities for some paracrine FGFs (e.g., FGF2) in the presence of HS [35, 49]. Thus, the canonical FGF signalling transduction unit is a stronger activator of intracellular signalling pathways than endocrine FGFs and may operate preferentially, or indeed override effects, in tissues exposed to both local and endocrine agonists.
FGF23:FGFR signalling in the absence of αKlotho
In the absence of αKl, FGF23 does show very weak binding towards FGFR1c, 2c, 3c and 4, with the highest affinities for FGFR2c and FGFR4 (Kd ~ 200 nM vs. 700 nM for FGFR1c), but not towards FGFRb isoforms [34]. At very high concentration, FGF23 has been shown to not only bind but also trans-activate downstream signalling pathways in several cell types proven to be deficient in αKl [34, 50, 51] (e.g., BaF3, HEK293, cardiac myocytes, renal myofibroblasts). The molecular detail of how FGF23 interacts with FGFRs in the absence of αKl remains to be clarified but is presumably potentiated by very high ligand concentrations found in some disease states. To date, αKl-independent FGF23 signalling has been reported in multiple cell types and tissues including the heart [51, 52], endothelium [53], liver [54], bone [55, 56] and parathyroid glands [57] as well as in hippocampal neurons [58], neutrophils [59, 60], pulmonary epithelia [61] and in fibroblasts derived from acutely injured kidneys [50] and fibrotic lung tissue [62]. In the case of the heart, liver, lungs and kidneys these effects appear mediated by FGFR4, whereas in neutrophils and osteoblasts, FGF23 is reported to activate FGFR2 and FGFR3, respectively. Effects on the endothelium, on the other hand, look to be transduced by FGFR1c, while in primary hippocampal cultures FGF23 is thought to act redundantly through multiple FGFRs. Consequently, independent of αKl, or possibly potentiated by its deficiency, FGF23 may participate is quite promiscuous interactions with different FGFRs depending on their local expression.
Interestingly of the endocrine FGFs, FGF23 has the highest residual affinity for HS, which may therefore be a universal requisite for FGF23:FGFR binding [35]. In tissues lacking αKl, alternate co-receptors may also participate in the signalling complex with FGFRs. This seems especially likely for FGFR4, which is thought to mediate some of the direct pathological effects of FGF23 in the heart and other tissues [52, 54, 63], as it has relatively reduced autophosphorylation and tyrosine kinase activity compared to other FGFRs and is consequently a relatively weak transducer of extracellular signals on its own [64, 65].Clearly much more work is needed to unravel the fine detail of how FGF23 can signal through FGFRs in an αKl-independent manner.
Soluble αKlotho is the shed ectodomain of transmembrane αKlotho
αKl is predominantly expressed in the renal distal convoluted tubules and the choroid plexus in the brain, with low-level expression in a number of other tissues including the proximal convoluted tubules, parathyroid glands, islet cells in the pancreas and the sinoatrial node [1, 66]. The physiological role of the relatively low-level expression of αKl in extra-renal tissues remains uncertain. Controversially, there is some suggestion that αKl may have a much wider tissue distribution than originally described [67], although enthusiasm for this possibility must be tempered by the reliance on suboptimal antisera or ultra-sensitive PCR techniques without confirmation at the level of the protein. These analyses are also confounded by the inability to distinguish full-length membrane-bound αKl and soluble protein. In a poorly understood process, αKl can be cleaved proximally at the cell surface (“α-cut”) by membrane-anchored sheddases ADAM10, ADAM17 and BACE1 [68, 69], generating a ‘soluble’ ~ 130 kDa isoform that is detectable in extracellular fluids (serum, urine and CSF) [4, 5], but indistinguishable in size from full-length membrane-bound αKl. Insulin has been identified as a potential stimulator of shedding [70], creating a theoretical feedback loop with cleaved αKl as a putative antagonist of IGF1/insulin signalling. In vitro, there is evidence that cleaved αKl can undergo further proteolysis with a cut between KL1 and KL2 domains (“β-cut”) [71], however, the 60–70 kDa fragments generated have not been convincingly detected in the systemic circulation. Since circulating αKl levels fall rapidly after bilateral nephrectomy, kidney- and tubule-specific ablation, as well as with inhibition of ectodomain shedding, soluble αKl is principally derived through cleavage of transmembrane protein in the distal convoluted tubules where it is most abundantly expressed [72, 73]. Indeed, distal tubule expression of αKl far exceeds that needed to support local FGF23-dependent signalling and therefore likely serves as an endocrine source of circulating ‘hormone’ through constitutive shedding [72].
In some mammals (but notably not rats) the C-terminus of exon 3 of the Kl gene carries an alternative splice donor site. In humans alternative mRNA splicing utilising this site would result in translation of a premature stop codon and a truncated form of the protein (~ 60 kDa) [4, 74]. This putative truncated protein comprises the KL1 domain and a unique 15aa C-terminal sequence but lacks the KL2 and transmembrane domains of the full-length gene product. In fact, the existence of a secreted isoform in mice and humans was originally only inferred on the basis that the splice variants encoded a signal sequence but no transmembrane domain [4, 74]. Since this shorter form also lacks the RBA-carrying KL2 domain it cannot sustain stable interactions with FGFR1c or tether the C-terminal FGF23 tails, which requires interactions with residues from both KL1 and KL2 [48]. Whether this splice variant harbours additional functions is uncertain, but appears to be largely homologous to an ancestral precursor found in some lower organisms that was allegedly involved in IGF1-related longevity pathways [75], but devoid of mineral regulating functions.
Regrettably, there is often a degree of ambiguity in the use of terminology for αKl, with ‘soluble’ and ‘secreted’ often used interchangeably, so it can be unclear which form of the protein is under investigation. Issues of identity are further compounded by a lack of specificity with even the best-characterised anti-αKl antisera (e.g., KM2076, where bands are even present in serum samples from the kl/kl mouse [76]). In particular, these yield a prominent non-specific band around ~ 60 kDa so confirmation of the truncated secreted form or cleaved αKl fragments is especially problematic by immunoassay. Currently there is no convincing evidence for its existence in humans and effects attributed to this splice variant may well lack any physiological relevance in mammals. Rather disconcertingly, other widely used monoclonal antibodies such as Ab181373 from Abcam gives a band for membrane αKl at lower molecular weight (~ 110 kDa) [67]. Serum αKl measurements are also subject to considerable variability which is at least partly due to very poor agreement between commercial ELISAs [77], where once again the provenance and analytical specificity of the antibodies utilised is ill-defined. The only reliable means of detecting and quantitating αKl appears to be through a low-throughput immunoprecipitation and western blotting procedure using two well-characterised monoclonal antibodies [76].
Recent reports cast doubt on the notion of a truncated secreted alternative human variant, showing that alternatively spliced mRNA is not translated but is a target for degradation through the nonsense-mediated decay RNA surveillance pathway [78]. Whether alternatively spliced mRNA serves a regulatory function has yet to be established. Regardless, it now seems that the cleaved ectodomain of membrane αKl is the only major soluble circulating form, at least in humans.
Soluble αKlotho can substitute for its transmembrane parent in complex with FGF23 and FGFR
Until quite recently it was widely believed that soluble αKl was essentially devoid of FGF23 co-receptor activity and instead mediated its many pleiotropic effects through an unidentified receptor or its intrinsic glycosidase activity [14, 38]; the latter altering the glycosylation, membrane occupancy and/or function of various ion channels and transporters [79–82]. Crucially, compelling new data now calls into question this hypothesis and prompts us to reappraise whether the myriad of seemingly unrelated functions, previously viewed as being discrete from those of αKl-dependent FGF23 mineral regulation, are truly independent of FGF23.
Several lines of evidence point to the fact that soluble αKl might participate in FGF23 signalling like its transmembrane parent. Initial clues came from in vitro studies showing that effects of soluble αKl were FGFR-dependent and in vivo studies where adenoviral-mediated over-expression of cleaved αKl in αKl-null mice stimulated the same canonical signalling pathways as those transduced by membrane αKl [83, 84]. Further evidence came from our own work showing that treatment of naturally αKl-deficient renal myofibroblasts with soluble αKl could effectively re-tune FGF23-driven signalling from pro-fibrotic NFAT to non-fibrotic canonical ERK/Egr1 pathways [63]. However, the recent studies of Chen et al. have provided a more direct answer to the question [48], showing at the atomic level that cleaved αKl possesses all the necessary molecular attributes to function as an “on-demand deliverable co-receptor” for FGF23 (Fig. 1).
While the 3 Å crystal structure unquestionably provides unique insight into the interactions of FGF23, FGFR1c and the αKl ectodomain, and compelling in vitro and in vivo functional data supports this modelling [48], several limitations to this approach exist. Typically, in order to achieve diffraction quality crystals, proteins are greatly concentrated, expressed in cells without mammalian glycosylating machinery, exposed to harsh unphysiological chemical conditions and modified genetically to promote crystallisation [85]. This is also the case for the crystallisation performed by Chen et al., where the FGF23–FGFR1c–αKl ternary complex was concentrated to 7 g/L and in which the FGF23 employed was not only a mutated to be resistant to cleavage (Arg to Gln mutations within the subtilisin-like proprotein convertase consensus recognition sequence; 176ArgHisThrArg179), but also lacked 46 residues from the C-terminus (Cys206 to Ile251) → 18% of the primary structure [48]. Although this region of the C-terminus appears dispensable for phosphaturic activity of FGF23 in vivo [86], an engagement with the FGFR–αKl receptor complex could conceivably engender some conformational differences. Ultimately the conformation of proteins that favours crystallisation may only represent one of several potential biological states and not necessarily the native hydrated configuration. The mixing stoichiometry of individual components of the complex may also result in alternate arrangements. In vivo, soluble αKl is present in 10–15 fold molar excess to FGF23, yet Chen et al. utilised mixing ratios in which FGF23 was added in slight excess to αKl (1.2:1) [48].
Indeed, the question arises that if cleaved αKl were to readily substitute for membrane αKl as an FGFR1c-binding partner, how are tissue-specific responses achieved given the almost ubiquitous expression of FGFRs and presence of soluble αKl in extracellular fluid? These observations would appear at odds with the prevailing notion that the comparatively limited tissue expression of membrane αKl defines the physiological targets of for FGF23 action [3, 46]. However, while soluble αKl might readily substitute for membrane αKl in terms of forming a high affinity FGF23 binding complex with FGFR1c, the ability of soluble αKl to activate downstream signalling compared to its membrane-tethered parent is presently unclear The in vitro studies of Chen et al. imply an equivalence in the ability of soluble αKl and transmembrane αKl to support FGF23-induced ERK phosphorylation [48], but this is based on concentrations of αKl and FGF23, approximately 200-fold and 1000-fold than their respective concentrations in plasma. This is also incongruent with earlier studies, which reported that the αKl ectodomain only supported very weak signal transduction, compared to membrane-tethered αKl [46]. Therefore, while it is plausible that soluble αKl might substitute for its transmembrane parent at sites of expression (e.g., the renal tubules), where local concentrations may be much higher than in systemic circulation, it is unlikely to participate in quantitatively relevant levels of complex formation elsewhere. Thus, in our view, tissue-specific expression of transmembrane αKl remains the major factor delineating the specificity of FGF23 signalling physiologically. Further work will therefore be needed to clarify the physiological significance of soluble αKl-mediated FGF23–FGFR signalling and elucidate the relevance of other ligand-receptor complexes that may participate in this endocrine axis.
αKlotho is a putative lectin not a glycan-cleaving enzyme
KL1 and KL2 domains both share sequence homology with β-glucosidases (E.C. 3.2.1.21). αKl is classified as a member of the glycoside hydrolase family 1(GH1) [87], which in humans includes lactase-phlorizin hydrolase (LPH), β-Klotho (a homologue of αKl that serves as a co-receptor for FGF21), cytoplasmic β-glycosidase, and Klotho-LPH-related- protein (KLPH). αKl shows the greatest similarity to LPH (~ 21% identity) [1], a single-pass membrane-tethered enzyme found in the brush border epithelial cells of the mammalian intestine, which breaks down lactose to galactose and glucose. However, the putative active site of KL1 and KL2 domains are atypical and diverge from those of true glycosidic enzymes, retaining only one of two conserved glutamate residues in either the catalyst acid/base or nucleophile position that are indispensable for activity [88, 89]. Consequently αKl is predicted to be inactive towards typical β-glucosidase substrates.
In this regard, the report by Chen et al. seemingly delivered another decisive finding; that the atomic structure of αKl was incompatible with the protein having significant glycosidase activity since both KL domains not only lack key catalytic residues but have active sites inaccessible to substrate or in a configuration having very low binding affinity [48]. Indeed, the purified αKl ectodomain, even when tested at excessively high concentration (~ 106 times physiological levels), failed to hydrolyse substrates for sialidase and β-glucuronidase. Taken together, this challenges the notion that αKl mediates any of its effects by acting as a sugar-cleaving enzyme as previously implicated in the activation of TRPV5, ROMK1 and inactivation of NaPi2a [79–81, 90]. In fact, it should be noted that in the majority of these studies, the mechanism of action was inferred through the nullifying effect of various chemical glycosidic inhibitors. Even in those few studies where activity was directly assayed, hydrolysis of substrate occurred at very low rates compared with bone fide glycosidases [91]. Since such activity now appears irreconcilable with the structure of αKl, it suggests that prior studies may have been confounded by contaminating proteins, or that effects were indirect via an as yet unidentified downstream glycan-cleaving mediator. Yet this is in stark contrast to the findings of multiple independent laboratories that report effects of soluble αKl without FGF23 and FGFRs [14, 20, 79, 81, 90, 92–94]. Indeed, an important caveat to assumptions of function based on such crystal structures is that the single rigid conformation of proteins within the complex imposed by lattice packing constraints is not necessarily representative of all possible biological states of the free unbound protein in which there is almost certainly a greater degree of structural flexibility. For instance, Chen et al. cite the positioning of the β6α6 loop which occludes substrate access to the putative KL1 active site while in complex with FGF23 and FGFR1c as one reason why αKl cannot cleave glycosides [48]. However, in silico modelling in the absence of either FGF23 or FGFR suggests that alternate, more open, conformations may exist with relatively minor movements in the KL1 β6α6 loop allowing access of some glyosidic moieties to the binding pocket [95].
Hence, one possibility, not incongruent with these observations, is that soluble αKl—while not acting enzymatically—may retain some residual sugar binding capacity [95]. In other words, αKl may function as a lectin. Consistent with this modelling, in vitro experiments show that soluble αKl can bind the α2–3-sialyllactose moiety of monosialoganglioside-enriched lipid rafts, the consequent reorganisation of which is thought to down-regulate raft-dependent PI3K signalling via effects on DAG [96]. Energetic considerations suggest that in the case of its interaction with α2–3-sialyllactose, it is preferentially bound by residues within the KL1 domain [94, 96]. Such lectin activity may account for some of the previously reported anti-proliferative and anti-fibrotic effects of soluble αKl on IGF1 and TRCP6 signalling that are both regulated by PI3K. Although functional data clearly supports this modelling, structural studies are needed to confirm the true nature of these interactions.
What can αKlotho do without FGF23?
Just as FGF23 has putative effects independent of αKl, there is an established literature of effects attributed to soluble αKl that occur independent of its cognate ligand and its membrane-tethered parent. Indeed, the effects of administering pharmacological doses of FGF23 or soluble αKl are undeniably quite distinct. That ancestral forms of αKl, equivalent in sequence to the KL1 domain of full-length αKl, not only predate the emergence of endocrine FGF23 but are also present in organisms without an apatite-based endoskeleton adds weight to notion of ligand-independent functions unrelated to mineral regulation [75, 97]. Critically, however, genetic ablation of FGF23 produces a very similar accelerated ageing phenotype to αKl-null animals [28]. Biochemically they are both characterised by elevated serum phosphate and 1,25(OH)2D concentrations and the phenotype of animals with either genetic deficiency can be improved by early restriction of dietary phosphate or ablation of the VDR [98, 99]. Likewise in humans, inactivating mutations in either αKl or FGF23 both result in hyperphosphataemic familial tumoral calcinosis, a rare debilitating condition characterised by recurrent periarticular calcifications in addition to other vascular and visceral manifestations (e.g., eyelid calcifications, arteriosclerosis, nephrocalcinosis, dental pulp stones) [100, 101]. Further experimental evidence for this co-dependence comes from the elegant genetic studies utilising double and triple knockout mice, where superimposing genetic αKl deficiency on FGF23-ablated animals, or combined ablation in mice with a non-functioning VDR, does not manifest in altered mineral handling compared to single Fgf23 and Klotho mutants or double knockout mutants (i.e. Klotho−/−/VDRΔ/Δ or Fgf23−/−/VDRΔ/Δ), respectively [102, 103].
Taken together this would appear to challenge the notion that αKl has significant physiological mineral regulating functions independent of FGF23, but instead suggests that they are likely to be mostly co-dependent. However, Fgf23−/− mice and αKl-null animals, while very similar—especially in respect to their mineral metabolism—do not appear identical in all aspects [1, 28]. Both genotypes have a shortened lifespan, retarded growth, infertility, hypoglycaemia, and, consistent with a reduction in peripheral lymphocytes, profound atrophy of the thymus. However, other ageing phenotypes appear unique to kl/kl mice such as arteriosclerosis, ectopic calcification in extra-renal tissues, skin atrophy, neuronal degeneration, and pulmonary emphysema. Indeed, αKl-null animals also have markedly elevated FGF23 concentrations compared to the expectedly undetectable levels in Fgf23−/− mice. Moreover, while left ventricular hypertrophy (LVH) is a reported feature of kl/kl mice [51, 104], it has not been reported in Fgf23-null animals. Finally, while both share abnormalities in bone histomorphometric indices suggesting low turnover, subtle differences are evident. It should be stressed, however, that a side-by-side comparison of Klotho−/− and Fgf23−/− mice bred on similar genetic backgrounds has only been undertaken in selected organs [103]. Nonetheless, consistent with the divergent effects of endogenous FGF23 and soluble αKl, the available evidence does point to global differences between the two mutant strains.
Despite this, genuinely FGF23 and FGFR independent actions are difficult to isolate in vivo due the ubiquitous expression of FGFR in most tissues and the presence of FGF23 in the extracellular fluid. However, perhaps the most definitive example of FGF23-independent actions comes from studies where soluble αKl was given to Fgf23−/− mice [81]. Here αKl was found to be acutely phosphaturic, consistent with its effects in vitro where it was shown to rapidly reduce NaPi2a abundance at the apical surface of immortalised tubule-like cells. However, a reduction in transport activity was also apparent in a vesicular and cell-free system, suggesting that αKl imparted direct changes in transporter function, independent of any signalling event. The authors’ surmised this gating effect could be due to alterations in NaPi2a glycosylation enacted by the sugar-modifying activity of αKl [81]. Given the report of Chen et al. [48], an alternative mechanism maybe needed to explain this observation. Although these experiments serve as proof-of-principle that αKl may have some intrinsic activity, the physiological relevance of such action in the context of an absolute deficiency of FGF23 remains questionable since this situation is seldom encountered biologically. In contrast, when Chen et al. administered soluble αKl to wild-type FGF23-replete mice, the phosphaturic effect was modest and almost completely absent with a mutant soluble αKl lacking the FGFR-interacting RBA [48].
Other molecular interactions of soluble αKl purportedly underpin its pleiotropic actions as an anti-fibrotic and anti-tumorigenic agent. These include, but are not limited to; binding to TGFβRII reducing its affinity for TGFβ1 [11]; binding to and sequestering Wnt1, Wnt3, Wnt4, Wnt5a and Wnt7a thereby inhibiting interaction with their cognate receptor complexes (LRP5/6/frizzled) [13, 20]; binding to and inhibiting IGFR1 antagonising downstream PI3K signalling [105]; and binding to TRPC1 and VEGFR2 stimulating VEGF-dependent endocytosis [106]. In the majority of instances binding domains are thought to reside in the KL1 domain, with the exception of TRPC1/VEGFR2 binding which is KL2-dependent, but have not been mapped to specific residues, or the protein complexes subjected to high resolution structural analyses. Thus, further work is needed to corroborate these findings using more specific techniques.
Although there is a degree of overlap between these pathways with respect to their biological endpoints, the likelihood of soluble αKl engaging substantively in all of these supposedly unique protein–protein interactions, in our view, would seem improbable. On the other hand, lectin activity towards sugar moieties on the gangliosides of lipid rafts may explain more promiscuous interactions and pleiotropic effects. In particular, there is convincing evidence from recent electrophysiological studies that soluble αKl can down-regulate TRPC6 calcium signalling through inhibitory effects on lipid-raft dependent exocytosis of the channel via a mechanism that is entirely independent of FGF23 and FGFRs [94]. Lending weight to its findings, and, in distinction to many other in vitro studies, the study by Wright et al. is one of the few to employ concentrations of soluble αKl that are similar to physiological levels, albeit in solutions free from other proteins. Indeed, it should be highlighted that much of the literature reports use of recombinant soluble αKl at concentrations of 1–10 nM while serum soluble αKl concentrations are typically only in the range 1–50 pM [76].
Recently, it has also been suggested, but not yet proven, that the flexible RBA of KL2 may facilitate binding to other receptors at the cell surface [107]. Alternative mechanisms may also account for equivalent actions in vivo with soluble αKl instead operating in a ligand-dependent manner; through competition with paracrine FGF binding; or through re-configuration of downstream signalling events either serving as a decoy receptor promoting more physiological canonical pathways [63, 108–110].
The role of FGF23–αKlotho signalling in CKD
While dysregulated FGF23–αKl signalling has been implicated in a number of mainly age-related pathologies, arguably the most compelling evidence for direct involvement in disease pathogenesis is found in the context of CKD [111–113]. Here, disturbances in the FGF23–αKl axis are characterised by a prodigious rise in FGF23 levels, but a reduction in αKl expression. These changes are evident at the earliest stages of CKD, and in the majority of individuals antedate those of known physiological mineral regulators such as PTH and 1,25(OH)2D [76, 114, 115]. That dysregulated FGF23–αKl signalling also appears consistently associated with unfavorable endpoints in this setting, has prompted intense interest in identifying other potential drivers of perturbations in this axis and is accordingly the focus of the remainder of this review.
The rise of FGF23 and fall of αKlotho in CKD
At least initially, αKl deficiency is not due to loss of functioning nephrons but is instead brought about by a rapid suppression of expression induced by injury evoked inflammatory cytokines, reactive oxygen species, uraemic toxins or RAAS activation (Fig. 2); mirroring the injurious pathways supposedly afforded protection by αKl (comprehensively reviewed by Neyra and Hu [116]). Somewhat perplexingly, therefore, αKl is lost at a time when it is needed the most. Epigenetic changes in the gene promoter such DNA hypermethylation and histone hypoacetylation have also been implicated as important drivers of repressed expression [44, 117]. Interestingly, promoter hypermethylation may also contribute to very low levels of αKl in kl/kl hypomorphic mice, in addition to disruption of promoter activity due to transgene insertion [1].
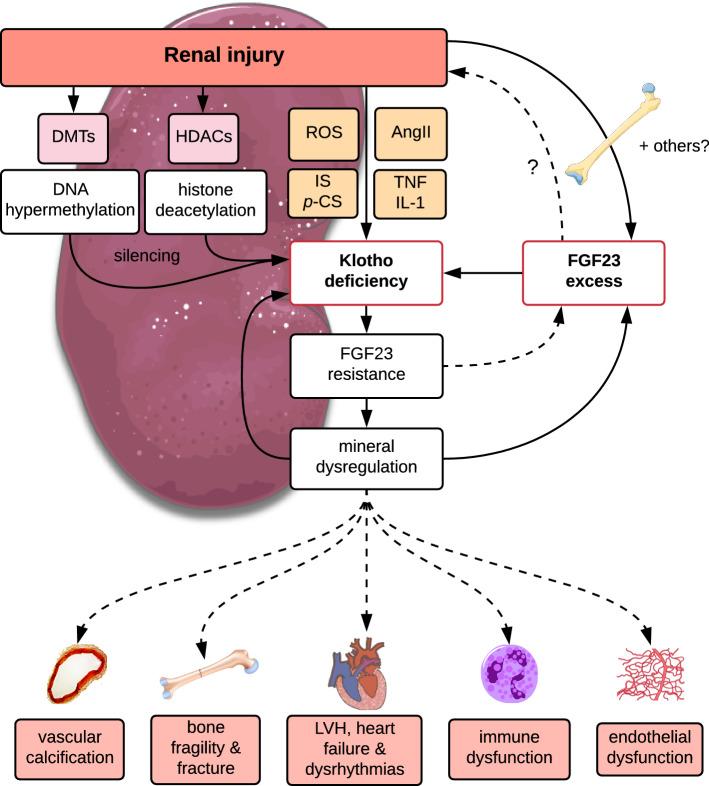
Fig. 2.
Hypothetical role of αKlotho deficiency in mineral dysregulation and pathological sequelae in CKD. In response to injury, the concerted action of epigenetic and non-epigenetic processes leads to silencing and suppression of αKl expression in the kidney. Renal injury also stimulates increased osteocytic synthesis and secretion of FGF23 with activation of inflammatory and hypoxia signalling pathways strongly implicated as key drivers of this response. FGF23 may suppress αKl expression through direct and indirect mechanisms. Ectopic FGF23 may also be expressed in extra-osseous non-physiological tissues (e.g., heart, liver, kidney) and supplement local and/or systemic levels. αKl deficiency results in end-organ resistance to FGF23 and failure in mineral homeostasis, which may feedback to bone to drive further increases in FGF23. Collectively and/or independently, loss of αKl, FGF23 excess and the consequent mineral dysregulation may promote the dysfunction in cardiovascular, bone and immune systems. Hypothetical pathways awaiting further experimental confirmation are indicated by dashed lines. AngII, angiotensin II; DMTs, dimethyltransferases; FGF23, fibroblast growth factor 23; HDACs, histone deacetylases; IL-1, interleukin-1β; IS, indoxyl sulphate; LVH, left ventricular hypertrophy; p-CS; cresyl sulphate; ROS, reactive oxygen species; TNF, tumour necrosis factor-α
Importantly, the loss of αKl may potentiate a state of end-organ resistance which feeds back to bone driving increased FGF23 synthesis (Fig. 2). Consequently, in a rudimentary fashion, elevated FGF23 levels may provide an integrated metric of dysfunction in glomerular and tubular kidney compartments. Yet, in patients with CKD stage 2, there is some evidence of a phase of relative phosphate wasting, and suppression of 1,25(OH)2D levels, consistent with functional over-activity of the FGF23–αKl axis rather than a secondary adaptive increase in FGF23 to offset reduced signalling efficacy [114, 118]. This argues against a state of early FGF23 resistance due to downregulated tubular αKl expression in which we would expect to find high phosphate and 1,25(OH)2D concentrations rather than apparent depletion. On the other hand, experimental studies in mice with more advanced CKD suggest that restoration of endogenous αKl expression modestly reduced circulating FGF23 levels [119] although this could also be ascribed to indirect effects on mineral biochemistry and bone phenotype. This is also consistent with prospective observational studies where following renal transplantation, a rise in soluble αKl was associated with a decline in circulating FGF23 [120]. In contrast, chronic delivery of a viral vector encoding a sequence equivalent to cleaved αKl in a murine model of diabetic renal disease, augmented FGF23 expression in bone and increased FGF23 in blood [121]. This suggests that soluble αKl might serve as a secretagogue for osteocytic FGF23. How appreciable amounts of circulating 130 kDa soluble αKl traverses from the vascular space through the lacunocanalicular system to where osteocytes reside is uncertain, since these proteoglycan-filled spaces create a molecular sieve that only allows passage of quite small molecule < ~ 70 kDa [122, 123]. This notion also seems inconsistent with the finding of extremely high levels of FGF23 in αKl-null animals. However, mice in these experiments achieve pharmacological levels of soluble αKl, which may differ in effects from the more modest differences in levels with intermittent peptide administration or endogenous changes after transplantation. Indeed, other reports in which soluble αKl was administered via tail-vein injection of a DNA plasmid to 5/6 nephrectomised heterozygous kl/+ mice showed no difference in FGF23 levels compared to injection of empty vector [92].
It is important to note that CKD is a state of partial, not absolute αKl deficiency and the threshold for effects of FGF23 on phosphate excretion and vitamin D synthesis may differ, permitting a dissociation of effects. This may allow FGF23-mediated suppression of Cyp27b, the rate-limiting enzyme for 1,25(OH)2D synthesis, but not adequate phosphate excretion. Thus, although some studies have suggested that a reduction in αKl might be one step further upstream from increments in FGF23, a clear delineation of the temporal sequence is still lacking. While the direct inhibitory effect of FGF23 on renal αKl expression needs to be substantiated further, elevated levels of FGF23 may also exacerbate αKl suppression indirectly via effects on 1,25(OH)2D. Regardless, what is clear is that the reciprocal changes in FGF23 and αKl observed consistently in animal models and human CKD alike appear inextricably entwined.
Is there a causal role for disturbances of the FGF23–αKlotho axis in complications associated with CKD?
Deregulation of the FGF23–αKl signalling axis, especially higher circulating FGF23 concentrations, have been robustly associated with poor outcomes in multiple observational CKD cohorts (as reviewed in Refs. [124, 125]). Data concerning the prognostic implications of soluble αKl levels are more equivocal [2], but may be underestimated due to the aforementioned issues with analytical performance of commercial soluble αKl assays. While elevated FGF23 levels have been linked to multiple cardiovascular and even non-cardiovascular endpoints, higher FGF23 levels would appear most consistently associated with the prevalence and severity of left ventricular hypertrophy (LVH), dysrhythmias and heart failure than other risk pathways [51, 126]. The biological plausibility of these findings is greatly reinforced by evidence from in vitro and preclinical studies in CKD mice, where very high FGF23 levels have been causally linked to the development of LVH and cardiac fibrosis [51, 52, 104, 127]. Although epidemiological data suggests that even modest increments in FGF23 are associated with similar endpoints in the non-CKD population, LVH is not a reported feature of humans with X-linked hypophosphataemia [128, 129], a condition of primary FGF23 excess or its murine homolog Hyp [130–132]. Thus, although FGF23 can induce hypertrophic gene programs in isolated cardiomyocytes, other factors in CKD associated with LVH (anaemia, hypertension and other haemodynamic changes, uraemic toxins, αKl deficiency) may be needed to potentiate FGF23′s actions on the heart in vivo. Importantly, there is also tentative evidence suggestive of a benefit with FGF23 lowering. In a post hoc secondary analysis of a placebo-controlled RCT of the calcimimetic cinacalcet in dialysis patients with severe secondary hyperparathyroidism (the EVOLVE study), reductions in FGF23 seen with cinacalcet treatment were associated with a relative reduction in the risk of cardiovascular events and mortality [133]. While certainly not evidence of causality, it represents one of the only examples of where changes in a mineral marker could be associated with an improvement in patient risk.
Pathways linking changes in FGF23–αKlotho signalling with pathology in CKD
Several pathways have been put forward to account for this apparent causal relationship, none of them mutually exclusive, wherein FGF23 is thought to operate in either a αKl-dependent or αKl-independent manner (Fig. 3). The proposed αKl-dependent pathways invoke canonical FGF23 signalling in the kidney resulting in (1) excessive sodium retention [29], (2) suppression of angiotensin-converting enzyme 2 and activation of RAAS [43, 134] or (3) loss of the direct cardioprotective effects of soluble αKl on the heart [135], and possibly other tissues, as a consequence of suppressed production in the kidney. However, these explanations remain contentious, as canonical FGF23 signalling if anything is impaired in CKD due to partial deficiency of αKl resulting in end-organ resistance. Indeed, recent epidemiological data does not consistently support this supposition in relation to effects on blood pressure and sodium excretion [136, 137]. Experimental animal data also points to direct antagonistic effects of soluble αKl on LVH via inhibition of Ca2+–TRPC6-dependent signalling [135], which at least in vitro do genuinely appear independent of both FGF23 and FGFR [94].
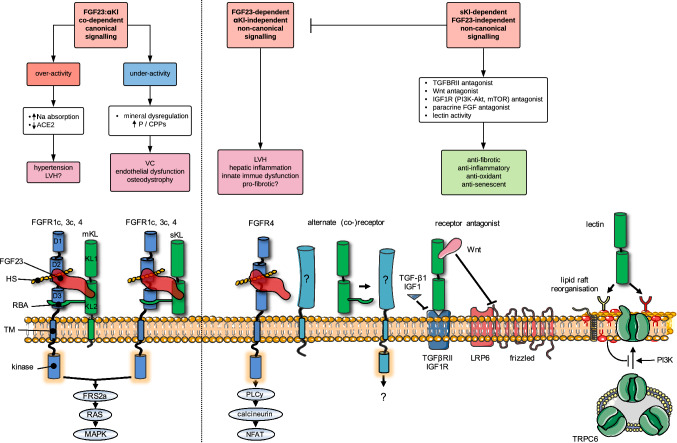
Fig. 3.
Proposed FGF23-dependent and FGF23-independent mechanisms of action of αKl and their links to pathologic signalling in CKD. Flow diagram depicts canonical (left) and non-canonical (right) pathways that may link disturbances in the FGF23:αKl axis with pathological endpoints. Molecular interactions at the cell surface that may underpin these pathways are illustrated underneath. Canonical FGF23:αKl signalling utilising either membrane-tethered αKl (mKl) or the soluble cleaved ectodomain (sKl) in complex with FGFR1c signals via the FRS2a–RAS–MAPK pathway. FGFR3c and FGFR4 may also have minor redundant roles. Over-activity of this pathway may lead to excessive sodium reabsorption, down-regulation of ACE2, hypertension and LVH. On the other hand, after loss of αKl, relative underactivity of this pathway may result in mineral dysregulation characterised by hyperphosphataemia and/or phosphate-related mineral aggregates (calciprotein particles, CPPs [174, 175]) and attendant vascular sequelae. Potentiated by the very high levels in CKD, FGF23 may also signal independently of αKl, directly driving pathology in the heart, kidney and liver. Here αKl-independent signalling is thought to occur through FGFR4 (although alternate co-receptors/co-factors may also be involved) via PLCy-calcium-dependent calcineurin-NFAT pathways. Silencing of αKl in CKD may contribute to pathological signalling through several mechanisms. Firstly, loss of soluble αKl as a decoy receptor potentiating off-target FGF23–FGFR4 or paracrine FGF signalling; secondly loss of direct protective effects of soluble αKl as a TGF-βRII or IGF1 receptor antagonist or via sequestration of Wnt proteins; thirdly, loss of homeostatic functions as a lectin, which binds α2,3-sialyllactose-containing monosialogangliosides (red lipid heads) clustered in lipid rafts and inhibits PI3K-dependent exocytosis of TRPC6. For simplicity the cartoons depict the FGF23–FGFR1c–αKl–HS in 1:1:1:1 stoichiometry rather than the 2:2:2:2 quaternary signalling complex predicted by Chen et al. [53]. D1–D3 denote immunoglobulin-like domains of FGFR. KL1 and KL2 denote tandem internal homologous KL repeats 1 and 2. ACE2, angiotensin I converting enzyme 2; Akt, serine/threonine-protein kinase; CPPs, calciprotein particles; FGF, fibroblast growth factor; FGFR, (FGF) receptor; FRF2a, fibroblast growth factor receptor substrate 2; HS, heparan sulphate; IGF1R, insulin-like growth factor 1 (IGF-1) receptor; LRP, low-density-lipoprotein-related protein; LVH, left ventricular hypertrophy; mKl, membrane αKlotho; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; Na, sodium; NFAT, nuclear factor of activated T-cells; P, phosphate; PI3K, phosphatidylinositol 3-kinases; PLCy, phospholipase C, gamma; RBA, receptor binding arm; sKl, soluble αKlotho; TGF, transforming growth factor; TGFBRII, TGF-β receptor II; TM, transmembrane domain; TRPC, transient receptor potential channel; VC, vascular calcification; Wnt, wingless integrated
Alternatively, FGF23 may induce maladaptive signalling pathways through comparatively promiscuous interactions with FGFRs independent of αKl. A prominent example of this includes the induction of hypertrophic programs in the cardiomyocyte via activation of FGFR4, which in vivo has been linked with development of LVH and cardiac fibrosis [52, 138]. Analogous to these findings in the heart, our own work in αKl-deficient injury-primed renal myofibroblasts, showed that treatment with soluble αKl antagonised FGF23–FGFR4–TRPC6–PLCy-dependent pro-fibrotic signalling but this effect was due to re-tuning FGF23 signalling towards more physiological ERK/Egr1 pathways rather than αKl mitigating effects in an independent manner [63]. A similar phenomenon has been described in work on hippocampal neurons, where co-administration of FGF23 and αKl was observed to modify downstream signalling from purely PLCy-dependent pathways with FGF23 alone, to mixed PLCy- and Akt-signalling in the presence of both [58]. While αKl had little effect on the inhibition of neuronal ramification by FGF23, it abolished the inductive effect of FGF23 on synapse density. Given that dysregulated FGF23 signalling has now been implicated in pathological pathways across multiple tissues (heart, bone, brain, kidneys, lung, endothelium, neutrophils etc.), pleiotropy provides a biologically plausible explanation for why FGF23 might predict both cardiovascular and non-cardiovascular endpoints in patients with CKD [139].
Thus, loss of αKl may not only create a degree of end-organ resistance to FGF23 in target tissues, but also potentiate the activation of deleterious off-target non-canonical signalling pathways by FGF23 in a more systemic fashion (Fig. 2). These are likely to be two of the major routes by which αKl deficiency might contribute to cardiotoxicity and other pathologies in CKD. However, this rather FGF23–αKl centric thinking overlooks the possible importance of the mineral dysregulation itself that occurs partly as consequence of disturbances in this axis. Indeed, many of the affects attributed to αKl deficiency in vivo can be recapitulated in vitro with the addition of excess mineral to the culture media.
A further complication is that FGF23 expression appears upregulated in tissues like the heart [140, 141], where high circulating levels are proposed to be directly pathogenic. So, the striking association of FGF23 with LVH may be one of reverse causation, where elevated FGF23 concentrations may partly be a consequence of cardiac dysfunction rather than cause. Whether ectopic production in heart or other extra-osseous tissues represents an adaptive response to injury/remodelling, is directly involved in pathogenesis or indeed augments circulating levels appreciably relative to osseous synthesis is unknown. What is clear is that the answer to these questions of causality lies in further experimentation, and it is unlikely that further epidemiologic machinations will help define these issues. Nonetheless, we still await evidence in human CKD that interventions aimed at specifically lowering FGF23, blocking pathological signalling or elevating αKl, offer benefit to the patient and an improvement in cardiovascular and renal outcomes.
Therapeutic targeting of the FGF23–αKlotho axis in experimental CKD
Advances in novel therapies targeting disturbances in the FGF23–αKl signalling axis appear promising.
FGF23 blockade
In rare hereditary hypophosphataemic disorders of FGF23 excess, such as those with X-linked hypophosphataemia, FGF23 blockade (burosumab) looks to be an effective therapeutic addition for biochemical control and improvement in the severity of rickets [142, 143]. Use of these agents in CKD is more problematic given the fine line between the protective effects of increased FGF23 that maintains phosphate balance in the face of nephron loss and possibly harmful off-target effects of FGF23 excess. In animal models of CKD, FGF23 blockade results in worsening mineral balance and attendant vascular sequelae [144]. Thus, if it does exist, the therapeutic window for FGF23 blockade in CKD would seem narrow and tricky to identify.
Restoration of αKlotho
That silencing of αKl occurs so early on in disease and that over-expression of αKl effectively reversed the phenotype and extended the life span of αKl-deficient mice has inevitably led to interest in whether it may be amenable to restorative therapies. To date, a number of studies have addressed this possibility either with pharmaceuticals that enhance endogenous αKl expression by targeting epigenetic (e.g., with DNA/histone demethylase activation [145–147] or histone deacetylase inhibition [148, 149]) and non-epigenetic mechanisms of suppression (e.g., PPARγ agonists [149], vitamin D sterols [150, 151], resveratrol [152], angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, statins [153], erythropoietin [154], rapamycin [155]), or with more specific approaches including transgenic insertion into the genome [14] and delivery as naked plasmid/virally encapsulated cDNA [83, 92, 156]. As a more readily translatable and reproducible strategy to raise systemic soluble αKl concentrations, exogenous recombinant peptide itself has also been administered (comprehensively reviewed elsewhere [157, 158]).
So far results in preclinical models are encouraging showing that restoration of endogenous expression can ameliorate kidney and bone disease progression in a number of CKD rodent models. However, while consistent with the theoretical benefit of boosting αKl, the improvement in the phenotype of these animals cannot be specifically attributed to the de-repression of αKl expression given the broad effects of the pharmacological agents used. Furthermore, efficacy is likely to be limited in patients with advanced disease where the reduced functioning renal mass is unlikely to support significantly increased production.
More specific interventions through delivery and forced over-expression of Klotho to various animal models of (generally severe) renal disease (unilateral ureteric obstruction (UUO), ischemia reperfusion injury (IRI), remnant kidney, streptozotocin-induced diabetic nephropathy, cyclosporin A nephropathy and adriamycin nephropathy) all show reduced renal fibrosis, although effect sizes differ quite markedly [13, 159–162]. Other studies show improvement in cardiac fibrosis in the remnant kidney model and in aged mice fed a high phosphate diet but not in angiotensin II-treated mice where over-expression resulted in marginally increased fibrosis [92, 104, 163]. Since these vectors result in increased membrane-bound αKl expression and pharmacological increases in soluble αKl concentrations it is not possible to delineate whether beneficial effects on fibrosis and due to one form of the protein or both. By contrast, chronic delivery of DNA encoding exclusively soluble αKl by viral vector in rodents with diabetic nephropathy (db/db-eNOS−/− mice) resulted in decreased serum phosphate concentrations and reduced aortic calcification, but ostensibly no improvement in renal function or pathology [83].
Supplementation with αKl peptide has also been shown to attenuate LPS-induced kidney injury and reduce blood pressure and albuminuria in db/db mice [164–167]. Consistent with findings in animals overexpressing αKl, intraperitoneal administration of peptide also improves renal fibrosis in UUO and bilateral IRI models [11, 108, 119, 159, 168, 169] and cardiac fibrosis in mice following isoproterenol-induced injury [119, 170]. Finally, and perhaps most impressively, administration of peptide is reportedly not only prophylactic for acute to chronic disease progression and development of post-AKI cardiomyopathy following IRI, but also therapeutic in animals with established injury. While these studies all appear consistent with the notion that targeting FGF23–αKl signalling may be efficacious in CKD, it remains unclear mechanistically how supplementation of αKl or de-repression of endogenous expression yields such benefit since all these models also engender a state of FGF23 excess. Consequently, further experimentation will be needed to elucidate whether αKl is working through direct cellular protection independent of FGF23, or through restoration physiological FGF23–αKl signalling networks. A further technical challenge relates to the relatively short plasma half-life of circulating αKl (~ 7 h in normal rats [73]), which may necessitate modification of native protein to prolong half-life and facilitate less frequent dosing strategies. Theoretically, over-replacement of αKl may also have undesired effects resulting in hypophosphataemia, hypocalcaemia and insulin resistance as seen in patients with rare genetic disorders of αKl excess [121, 171]. Nevertheless, supplementation of recombinant αK looks to hold considerable promise and remains the only means of delivery by which therapeutic dose can be accurately controlled, and with it, a more consistent anticipated effect.
Conclusions
This review has discussed the existing body of knowledge and controversies surrounding the molecular basis of how soluble αKl works. Although an ever-increasing number of studies report effects of αKl that are independent of FGF23, and vice versa, some recent findings have instead implied a broad co-dependence of actions not only at the level of the transmembrane receptor but also the shed ectodomain. Accounting for the many biological effects attributed to this rather enigmatic protein is as yet difficult to reconcile. Unravelling this conundrum has particular relevance to CKD, where dysregulation of FGF23–αKl signalling appears central to the pathogenesis of mineral, cardiovascular and bone disorders. Despite the prevalence of these disorders in this population, there are no effective therapies. Irrespective of the true extent of the inter-dependency of FGF23 and αKl actions, given that the early loss of αKl is an almost universal feature of CKD it is a prime target for replacement or restoration. We eagerly await the next steps when αKl-based therapeutics are trialled in humans.
Acknowledgements
The authors were supported by a Grant-in-Aid (GIA-021-2017) from the RMH Home Lottery Research Fund. The figures in this review were partly generated using vector images freely available from Servier Medical Art (http://smart.servier.com) which is licensed under a Creative Commons Attribution 3.0 Unported License.
Author contributions
ERS, TDH: manuscript drafting, revision and final approval.
Compliance with ethical standards
Conflict of interest
The authors declare they have no relevant conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Tan SJ, Smith ER, Hewitson TD, Holt SG, Toussaint ND. The importance of klotho in phosphate metabolism and kidney disease. Nephrology (Carlton) 2014;19:439–449. doi: 10.1111/nep.12268. [DOI] [PubMed] [Google Scholar]
- 3.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 5.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.m509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 8.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/bc.2008.028. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, Wang S, Xiao T, Xu X, He T, Xia X, Wang J, Zhao J. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol. 2015;26:2434–2446. doi: 10.1681/asn.2014060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.m110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012;303:F1641–F1651. doi: 10.1152/ajprenal.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/asn.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullah M, Sun Z. Klotho deficiency accelerates stem cells aging by impairing telomerase activity. J Gerontol A Biol Sci Med Sci. 2018 doi: 10.1093/gerona/gly261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW. alpha-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L566–L575. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. 2014;85:855–870. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, Takemura Y, Ohishi M, Katsuya T, Rakugi H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–346. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 19.Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, Oguro R, Yamamoto-Hanasaki H, Tatara Y, Takeya Y, Rakugi H. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int. 2011;11:510–516. doi: 10.1111/j.1447-0594.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 21.Kuro-o M, Hanaoka K, Hiroi Y, Noguchi T, Fujimori Y, Takewaki S, Hayasaka M, Katoh H, Miyagishi A, Nagai R, et al. Salt-sensitive hypertension in transgenic mice overexpressing Na(+)-proton exchanger. Circ Res. 1995;76:148–153. doi: 10.1161/01.RES.76.1.148. [DOI] [PubMed] [Google Scholar]
- 22.Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–1283. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen TH, Kuro OM, Chen CH, Sue YM, Chen YC, Wu HH, Cheng CY. The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol. 2013;698:67–73. doi: 10.1016/j.ejphar.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Smith ER. Untangling the thread of life spun by αKlotho. J Mol Med. 2018;96:857–859. doi: 10.1007/s00109-018-1671-4. [DOI] [PubMed] [Google Scholar]
- 25.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Itoh N, Ohta H, Konishi M. Endocrine FGFs: evolution, physiology, pathophysiology, and pharmacotherapy. Front Endocrinol (Lausanne) 2015;6:154. doi: 10.3389/fendo.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/jbmr.0301264. [DOI] [PubMed] [Google Scholar]
- 28.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Investig. 2004;113:561–568. doi: 10.1172/jci19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6:744–759. doi: 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 2014;33:229–246. doi: 10.1002/embj.201284188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/mcb.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuro-O M, Moe OW. FGF23-alphaKlotho as a paradigm for a kidney-bone network. Bone. 2017;100:4–18. doi: 10.1016/j.bone.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Turan K, Ata P. Effects of intra- and extracellular factors on anti-aging klotho gene expression. Genet Mol Res. 2011;10:2009–2023. doi: 10.4238/vol10-3gmr1261. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, Kerr Whitfield G. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414:557–562. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N. Klotho is a target gene of PPAR-gamma. Kidney Int. 2008;74:732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 41.Tang R, Zhou QL, Ao X, Peng WS, Veeraragoo P, Tang TF. Fosinopril and losartan regulate klotho gene and nicotinamide adenine dinucleotide phosphate oxidase expression in kidneys of spontaneously hypertensive rats. Kidney Blood Press Res. 2011;34:350–357. doi: 10.1159/000326806. [DOI] [PubMed] [Google Scholar]
- 42.de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin–angiotensin–aldosterone system and vitamin D-FGF-23–klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603–1609. doi: 10.1681/asn.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/asn.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiyama A, Arai F, Sakai D, Yokoyama K, Mochida J. The effects of oxygen tension and antiaging factor Klotho on Wnt signaling in nucleus pulposus cells. Arthritis Res Ther. 2012;14:R105. doi: 10.1186/ar3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.c500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goetz R, Ohnishi M, Ding X, Kurosu H, Wang L, Akiyoshi J, Ma J, Gai W, Sidis Y, Pitteloud N, Kuro OM, Razzaque MS, Mohammadi M. Klotho coreceptors inhibit signaling by paracrine fibroblast growth factor 8 subfamily ligands. Mol Cell Biol. 2012;32:1944–1954. doi: 10.1128/mcb.06603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M. alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–466. doi: 10.1038/nature25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goetz R, Ohnishi M, Kir S, Kurosu H, Wang L, Pastor J, Ma J, Gai W, Kuro-o M, Razzaque MS, Mohammadi M. Conversion of a paracrine fibroblast growth factor into an endocrine fibroblast growth factor. J Biol Chem. 2012;287:29134–29146. doi: 10.1074/jbc.m112.342980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith ER, Tan SJ, Holt SG, Hewitson TD. FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci Rep. 2017;7:3345. doi: 10.1038/s41598-017-02709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Investig. 2011;121:4393–4408. doi: 10.1172/jci46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstadt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22:1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silswal N, Touchberry CD, Daniel DR, McCarthy DL, Zhang S, Andresen J, Stubbs JR, Wacker MJ. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab. 2014;307:E426–E436. doi: 10.1152/ajpendo.00264.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M, Wolf M, Faul C. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90:985–996. doi: 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murali SK, Roschger P, Zeitz U, Klaushofer K, Andrukhova O, Erben RG. FGF23 regulates bone mineralization in a 1,25(OH)2 D3 and Klotho-independent manner. J Bone Miner Res. 2016;31:129–142. doi: 10.1002/jbmr.2606. [DOI] [PubMed] [Google Scholar]
- 56.Murali SK, Andrukhova O, Clinkenbeard EL, White KE, Erben RG. Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp mice. PLoS Biol. 2016;14:e1002427. doi: 10.1371/journal.pbio.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olauson H, Lindberg K, Amin R, Sato T, Jia T, Goetz R, Mohammadi M, Andersson G, Lanske B, Larsson TE. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet. 2013;9:e1003975. doi: 10.1371/journal.pgen.1003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hensel N, Schon A, Konen T, Lubben V, Forthmann B, Baron O, Grothe C, Leifheit-Nestler M, Claus P, Haffner D. Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density. J Neurochem. 2016;137:756–769. doi: 10.1111/jnc.13585. [DOI] [PubMed] [Google Scholar]
- 59.Yang K, Peretz-Soroka H, Wu J, Zhu L, Cui X, Zhang M, Rigatto C, Liu Y, Lin F. Fibroblast growth factor 23 weakens chemotaxis of human blood neutrophils in microfluidic devices. Sci Rep. 2017;7:3100. doi: 10.1038/s41598-017-03210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstadt HJ, Meersch M, Unruh M, Zarbock A. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Investig. 2016;126:962–974. doi: 10.1172/jci83470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krick S, Grabner A, Baumlin N, Yanucil C, Helton S, Grosche A, Sailland J, Geraghty P, Viera L, Russell DW, Wells JM, Xu X, Gaggar A, Barnes J, King GD, Campos M, Faul C, Salathe M. Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J. 2018 doi: 10.1183/13993003.00236-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnes JW, Duncan D, Helton S, Hutcheson S, Kurundkar D, Logsdon NJ, Locy M, Garth J, Denson R, Farver C, Vo HT, King G, Kentrup D, Faul C, Kulkarni T, De Andrade JA, Yu Z, Matalon S, Thannickal VJ, Krick S. Role of fibroblast growth factor 23 and klotho cross talk in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2019;317:L141–L154. doi: 10.1152/ajplung.00246.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith ER, Holt SG, Hewitson TD. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-beta autoinduction. Int J Biochem Cell Biol. 2017;92:63–78. doi: 10.1016/j.biocel.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 64.Vainikka S, Joukov V, Wennstrom S, Bergman M, Pelicci PG, Alitalo K. Signal transduction by fibroblast growth factor receptor-4 (FGFR-4). Comparison with FGFR-1. J Biol Chem. 1994;269:18320–18326. [PubMed] [Google Scholar]
- 65.Wang JK, Gao G, Goldfarb M. Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol Cell Biol. 1994;14:181–188. doi: 10.1128/MCB.14.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 67.Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao LL, Hiemstra TF, Zehnder D. alpha-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100:E1308–E1318. doi: 10.1210/jc.2015-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Loon EP, Pulskens WP, van der Hagen EA, Lavrijsen M, Vervloet MG, van Goor H, Bindels RJ, Hoenderop JG. Shedding of klotho by ADAMs in the kidney. Am J Physiol Renal Physiol. 2015;309:F359–F368. doi: 10.1152/ajprenal.00240.2014. [DOI] [PubMed] [Google Scholar]
- 69.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, Abraham CR. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry. 2014;53:5579–5587. doi: 10.1021/bi500409n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25:2169–2175. doi: 10.1681/asn.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-o M, Moe OW. Renal production, uptake, and handling of circulating alphaKlotho. J Am Soc Nephrol. 2016;27:79–90. doi: 10.1681/asn.2014101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/S0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 75.Chateau MT, Araiz C, Descamps S, Galas S. Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging (Albany NY) 2010;2:567–581. doi: 10.18632/aging.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30:223–233. doi: 10.1093/ndt/gfu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, Vervloet MG, Consortium N Laboratory aspects of circulating alpha-Klotho. Nephrol Dial Transplant. 2013;28:2283–2287. doi: 10.1093/ndt/gft236. [DOI] [PubMed] [Google Scholar]
- 78.Mencke R, Harms G, Moser J, van Meurs M, Diepstra A, Leuvenink HG, Hillebrands JL. Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight. 2017 doi: 10.1172/jci.insight.94375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 80.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 83.Hum JM, O’Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, Bhaskaran M, Johnson RL, Wilson JM, Smith RC, White KE. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble klotho. J Am Soc Nephrol. 2017;28:1162–1174. doi: 10.1681/asn.2015111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89:140–150. doi: 10.1007/s00223-011-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wlodawer A, Minor W, Dauter Z, Jaskolski M. Protein crystallography for aspiring crystallographers or how to avoid pitfalls and traps in macromolecular structure determination. FEBS J. 2013;280:5705–5736. doi: 10.1111/febs.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23–FGFR–Klotho complex formation. Proc Natl Acad Sci USA. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tribolo S, Berrin JG, Kroon PA, Czjzek M, Juge N. The crystal structure of human cytosolic beta-glucosidase unravels the substrate aglycone specificity of a family 1 glycoside hydrolase. J Mol Biol. 2007;370:964–975. doi: 10.1016/j.jmb.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 88.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576:341–345. doi: 10.1016/S0167-4781(02)00281-6. [DOI] [PubMed] [Google Scholar]
- 89.Matern H, Boermans H, Lottspeich F, Matern S. Molecular cloning and expression of human bile acid beta-glucosidase. J Biol Chem. 2001;276:37929–37933. doi: 10.1074/jbc.m104290200. [DOI] [PubMed] [Google Scholar]
- 90.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279:9777–9784. doi: 10.1074/jbc.m312392200. [DOI] [PubMed] [Google Scholar]
- 92.Xie J, Yoon J, An SW, Kuro-o M, Huang CL. Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015;26:1150–1160. doi: 10.1681/asn.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13:254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 94.Wright JD, An SW, Xie J, Lim C, Huang CL. Soluble klotho regulates TRPC6 calcium signaling via lipid rafts, independent of the FGFR–FGF23 pathway. Faseb J. 2019 doi: 10.1096/fj.201900321r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wright JD, An SW, Xie J, Yoon J, Nischan N, Kohler JJ, Oliver N, Lim C, Huang CL. Modeled structural basis for the recognition of alpha2–3-sialyllactose by soluble Klotho. Faseb J. 2017;31:3574–3586. doi: 10.1096/fj.201700043r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dalton G, An SW, Al-Juboori SI, Nischan N, Yoon J, Dobrinskikh E, Hilgemann DW, Xie J, Luby-Phelps K, Kohler JJ, Birnbaumer L, Huang CL. Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci USA. 2017;114:752–757. doi: 10.1073/pnas.1620301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sugano Y, Lardelli M. Identification and expression analysis of the zebrafish orthologue of Klotho. Dev Genes Evol. 2011;221:179–186. doi: 10.1007/s00427-011-0367-3. [DOI] [PubMed] [Google Scholar]
- 98.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24:3562–3571. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/asn.2006121385. [DOI] [PubMed] [Google Scholar]
- 100.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7:318–319. [PubMed] [Google Scholar]
- 101.Ramnitz MS, Gourh P, Goldbach-Mansky R, Wodajo F, Ichikawa S, Econs MJ, White KE, Molinolo A, Chen MY, Heller T, Del Rivero J, Seo-Mayer P, Arabshahi B, Jackson MB, Hatab S, McCarthy E, Guthrie LC, Brillante BA, Gafni RI, Collins MT. Phenotypic and genotypic characterization and treatment of a cohort with familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome. J Bone Miner Res. 2016;31:1845–1854. doi: 10.1002/jbmr.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Erben RG. α-Klotho’s effects on mineral homeostasis are fibroblast growth factor-23 dependent. Curr Opin Nephrol Hypertens. 2018;27:229–235. doi: 10.1097/mnh.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrukhova O, Bayer J, Schuler C, Zeitz U, Murali SK, Ada S, Alvarez-Pez JM, Smorodchenko A, Erben RG. Klotho lacks an FGF23-independent role in mineral homeostasis. J Bone Miner Res. 2017;32:2049–2061. doi: 10.1002/jbmr.3195. [DOI] [PubMed] [Google Scholar]
- 104.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro OM, Hill JA, Moe OW. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26:1290–1302. doi: 10.1681/asn.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 106.Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, Sonomura K, Adachi Y, Shibuya M, Shirayama T, Tanda S, Hatta T, Sasaki S, Mori Y, Matsubara H. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci USA. 2010;107:19308–19313. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuro OM. The Klotho proteins in health and disease. Nat Rev Nephrol. 2018 doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 108.Guan X, Nie L, He T, Yang K, Xiao T, Wang S, Huang Y, Zhang J, Wang J, Sharma K, Liu Y, Zhao J. Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J Pathol. 2014;234:560–572. doi: 10.1002/path.4420. [DOI] [PubMed] [Google Scholar]
- 109.Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B, Wolf I. KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res. 2011;17:4254–4266. doi: 10.1158/1078-0432.ccr-10-2749. [DOI] [PubMed] [Google Scholar]
- 110.Richter B, Faul C. FGF23 actions on target tissues-with and without klotho. Front Endocrinol (Lausanne) 2018;9:189. doi: 10.3389/fendo.2018.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moe OW. Fibroblast growth factor 23: friend or foe in uremia? J Clin Investig. 2012;122:2354–2356. doi: 10.1172/jci64184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–747. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/asn.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neyra JA, Hu MC. alphaKlotho and chronic kidney disease. Vitam Horm. 2016;101:257–310. doi: 10.1016/bs.vh.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Azuma M, Koyama D, Kikuchi J, Yoshizawa H, Thasinas D, Shiizaki K, Kuro-o M, Furukawa Y, Kusano E. Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J. 2012;26:4264–4274. doi: 10.1096/fj.12-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pavik I, Jaeger P, Kistler AD, Poster D, Krauer F, Cavelti-Weder C, Rentsch KM, Wuthrich RP, Serra AL. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 2011;79:234–240. doi: 10.1038/ki.2010.375. [DOI] [PubMed] [Google Scholar]
- 119.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro OM, Moe OW. Recombinant alpha-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017;91:1104–1114. doi: 10.1016/j.kint.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tan SJ, Crosthwaite A, Langsford D, Obeysekere V, Ierino FL, Roberts MA, Hughes PD, Hewitson TD, Dwyer KM, Toussaint ND. Mineral adaptations following kidney transplantation. Transpl Int. 2017;30:463–473. doi: 10.1111/tri.12925. [DOI] [PubMed] [Google Scholar]
- 121.Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Investig. 2012;122:4710–4715. doi: 10.1172/jci64986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/S8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- 123.Knothe Tate ML. “Whither flows the fluid in bone?” An osteocyte’s perspective. J Biomech. 2003;36:1409–1424. doi: 10.1016/S0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 124.Smith ER. The use of fibroblast growth factor 23 testing in patients with kidney disease. Clin J Am Soc Nephrol. 2014;9:1283–1303. doi: 10.2215/cjn.10941013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith ER, McMahon LP, Holt SG. Fibroblast growth factor 23. Ann Clin Biochem. 2014;51:203–227. doi: 10.1177/0004563213510708. [DOI] [PubMed] [Google Scholar]
- 126.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/asn.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hao H, Li X, Li Q, Lin H, Chen Z, Xie J, Xuan W, Liao W, Bin J, Huang X, Kitakaze M, Liao Y. FGF23 promotes myocardial fibrosis in mice through activation of beta-catenin. Oncotarget. 2016;7:64649–64664. doi: 10.18632/oncotarget.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takashi Y, Kinoshita Y, Hori M, Ito N, Taguchi M, Fukumoto S. Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res. 2017;42:132–137. doi: 10.1080/07435800.2016.1242604. [DOI] [PubMed] [Google Scholar]
- 129.Chesher D, Oddy M, Darbar U, Sayal P, Casey A, Ryan A, Sechi A, Simister C, Waters A, Wedatilake Y, Lachmann RH, Murphy E. Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J Inherit Metab Dis. 2018;41:865–876. doi: 10.1007/s10545-018-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pastor-Arroyo EM, Gehring N, Krudewig C, Costantino S, Bettoni C, Knopfel T, Sabrautzki S, Lorenz-Depiereux B, Pastor J, Strom TM, Hrabe de Angelis M, Camici GG, Paneni F, Wagner CA, Rubio-Aliaga I. The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int. 2018;94:49–59. doi: 10.1016/j.kint.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 131.Liu ES, Thoonen R, Petit E, Yu B, Buys ES, Scherrer-Crosbie M, Demay MB. Increased circulating FGF23 does not lead to cardiac hypertrophy in the male Hyp mouse model of XLH. Endocrinology. 2018;159:2165–2172. doi: 10.1210/en.2018-00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leifheit-Nestler M, Richter B, Basaran M, Nespor J, Vogt I, Alesutan I, Voelkl J, Lang F, Heineke J, Krick S, Haffner D. Impact of altered mineral metabolism on pathological cardiac remodeling in elevated fibroblast growth factor 23. Front Endocrinol (Lausanne) 2018;9:333. doi: 10.3389/fendo.2018.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, Drueke TB, Herzog CA, London GM, Mahaffey KW, Wheeler DC, Stolina M, Dehmel B, Goodman WG, Floege J. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation. 2015;132:27–39. doi: 10.1161/circulationaha.114.013876. [DOI] [PubMed] [Google Scholar]
- 134.Pi M, Ye R, Han X, Armstrong B, Liu X, Chen Y, Sun Y, Quarles LD. Cardiovascular interactions between fibroblast growth factor-23 and angiotensin II. Sci Rep. 2018;8:12398. doi: 10.1038/s41598-018-30098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3:1238. doi: 10.1038/ncomms2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu H, Hashem A, Witasp A, Mencke R, Goldsmith D, Barany P, Bruchfeld A, Wernerson A, Carrero JJ, Olauson H. Fibroblast growth factor 23 is associated with fractional excretion of sodium in patients with chronic kidney disease. Nephrol Dial Transplant. 2018 doi: 10.1093/ndt/gfy315. [DOI] [PubMed] [Google Scholar]
- 137.Akhabue E, Montag S, Reis JP, Pool LR, Mehta R, Yancy CW, Zhao L, Wolf M, Gutierrez OM, Carnethon MR, Isakova T. FGF23 (fibroblast growth factor-23) and incident hypertension in young and middle-aged adults: the CARDIA study. Hypertension. 2018;72:70–76. doi: 10.1161/hypertensionaha.118.11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, Singh S, Wolf M, Hermann S, Stypmann J, Di Marco GS, Brand M, Wacker MJ, Faul C. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep. 2017;7:1993. doi: 10.1038/s41598-017-02068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, Landray MJ, Moe SM, Yang J, Holland L, di Giuseppe R, Bouma-de Krijger A, Mihaylova B, Herrington WG. Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: a meta-analysis. J Am Soc Nephrol. 2018;29:2015–2027. doi: 10.1681/asn.2017121334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsui I, Oka T, Kusunoki Y, Mori D, Hashimoto N, Matsumoto A, Shimada K, Yamaguchi S, Kubota K, Yonemoto S, Higo T, Sakaguchi Y, Takabatake Y, Hamano T, Isaka Y. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int. 2018;94:60–71. doi: 10.1016/j.kint.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 141.Richter M, Lautze HJ, Walther T, Braun T, Kostin S, Kubin T. The failing heart is a major source of circulating FGF23 via oncostatin M receptor activation. J Heart Lung Transplant. 2015;34:1211–1214. doi: 10.1016/j.healun.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 142.Insogna KL, Briot K, Imel EA, Kamenicky P, Ruppe MD, Portale AA, Weber T, Pitukcheewanont P, Cheong HI, Jan de Beur S, Imanishi Y, Ito N, Lachmann RH, Tanaka H, Perwad F, Zhang L, Chen CY, Theodore-Oklota C, Mealiffe M, San Martin J, Carpenter TO. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33:1383–1393. doi: 10.1002/jbmr.3475. [DOI] [PubMed] [Google Scholar]
- 143.Carpenter TO, Whyte MP, Imel EA, Boot AM, Hogler W, Linglart A, Padidela R, Van’t Hoff W, Mao M, Chen CY, Skrinar A, Kakkis E, San Martin J, Portale AA. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378:1987–1998. doi: 10.1056/NEJMoa1714641. [DOI] [PubMed] [Google Scholar]
- 144.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Investig. 2012;122:2543–2553. doi: 10.1172/jci61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Q, Liu L, Lin W, Yin S, Duan A, Liu Z, Cao W. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017;91:144–156. doi: 10.1016/j.kint.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 146.Yin S, Zhang Q, Yang J, Lin W, Li Y, Chen F, Cao W. TGFbeta-incurred epigenetic aberrations of miRNA and DNA methyltransferase suppress Klotho and potentiate renal fibrosis. Biochim Biophys Acta Mol Cell Res. 2017;1864:1207–1216. doi: 10.1016/j.bbamcr.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 147.Irifuku T, Doi S, Sasaki K, Doi T, Nakashima A, Ueno T, Yamada K, Arihiro K, Kohno N, Masaki T. Inhibition of H3K9 histone methyltransferase G9a attenuates renal fibrosis and retains klotho expression. Kidney Int. 2016;89:147–157. doi: 10.1038/ki.2015.291. [DOI] [PubMed] [Google Scholar]
- 148.Lin W, Li Y, Chen F, Yin S, Liu Z, Cao W. Klotho preservation via histone deacetylase inhibition attenuates chronic kidney disease-associated bone injury in mice. Sci Rep. 2017;7:46195. doi: 10.1038/srep46195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin W, Zhang Q, Liu L, Yin S, Liu Z, Cao W. Klotho restoration via acetylation of peroxisome proliferation-activated receptor gamma reduces the progression of chronic kidney disease. Kidney Int. 2017;92:669–679. doi: 10.1016/j.kint.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 150.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 151.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82:1261–1270. doi: 10.1038/ki.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hsu SC, Huang SM, Chen A, Sun CY, Lin SH, Chen JS, Liu ST, Hsu YJ. Resveratrol increases anti-aging Klotho gene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int J Biochem Cell Biol. 2014;53:361–371. doi: 10.1016/j.biocel.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 153.Kuwahara N, Sasaki S, Kobara M, Nakata T, Tatsumi T, Irie H, Narumiya H, Hatta T, Takeda K, Matsubara H, Hushiki S. HMG-CoA reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int J Cardiol. 2008;123:84–90. doi: 10.1016/j.ijcard.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 154.Sugiura H, Yoshida T, Mitobe M, Shiohira S, Nitta K, Tsuchiya K. Recombinant human erythropoietin mitigates reductions in renal klotho expression. Am J Nephrol. 2010;32:137–144. doi: 10.1159/000315864. [DOI] [PubMed] [Google Scholar]
- 155.Tataranni T, Biondi G, Cariello M, Mangino M, Colucci G, Rutigliano M, Ditonno P, Schena FP, Gesualdo L, Grandaliano G. Rapamycin-induced hypophosphatemia and insulin resistance are associated with mTORC2 activation and Klotho expression. Am J Transplant. 2011;11:1656–1664. doi: 10.1111/j.1600-6143.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 156.Shin YJ, Luo K, Quan Y, Ko EJ, Chung BH, Lim SW, Yang CW. Therapeutic challenge of minicircle vector encoding klotho in animal model. Am J Nephrol. 2019;49:413–424. doi: 10.1159/000499863. [DOI] [PubMed] [Google Scholar]
- 157.Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone. 2017;100:41–49. doi: 10.1016/j.bone.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mencke R, Olauson H, Hillebrands JL. Effects of Klotho on fibrosis and cancer: a renal focus on mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2017;121:85–100. doi: 10.1016/j.addr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 159.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC. alphaKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol. 2016;27:2331–2345. doi: 10.1681/ASN.2015060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jin M, Lv P, Chen G, Wang P, Zuo Z, Ren L, Bi J, Yang CW, Mei X, Han D. Klotho ameliorates cyclosporine A-induced nephropathy via PDLIM2/NF-kB p65 signaling pathway. Biochem Biophys Res Commun. 2017;486:451–457. doi: 10.1016/j.bbrc.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 161.Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin–angiotensin system. Am J Pathol. 2015;185:3211–3223. doi: 10.1016/j.ajpath.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Deng M, Luo Y, Li Y, Yang Q, Deng X, Wu P, Ma H. Klotho gene delivery ameliorates renal hypertrophy and fibrosis in streptozotocin-induced diabetic rats by suppressing the Rho-associated coiled-coil kinase signaling pathway. Mol Med Rep. 2015;12:45–54. doi: 10.3892/mmr.2015.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu X, Chen Y, McCoy CW, Zhao T, Quarles DL, Pi M, Bhattacharya SK, King G, Sun Y. Differential regulatory role of soluble klothos on cardiac fibrogenesis in hypertension. Am J Hypertens. 2016;29:1140–1147. doi: 10.1093/ajh/hpw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H. Klotho suppresses the renin–angiotensin system in adriamycin nephropathy. Nephrol Dial Transplant. 2017;32:791–800. doi: 10.1093/ndt/gfw340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H. Klotho ameliorates medullary fibrosis and pressure natriuresis in hypertensive rat kidneys. Hypertension. 2018;72:1151–1159. doi: 10.1161/hypertensionaha.118.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jou-Valencia D, Molema G, Popa E, Aslan A, van Dijk F, Mencke R, Hillebrands JL, Heeringa P, Hoenderop JG, Zijlstra JG, van Meurs M, Moser J. Renal klotho is reduced in septic patients and pretreatment with recombinant klotho attenuates organ injury in lipopolysaccharide-challenged mice. Crit Care Med. 2018;46:e1196–e1203. doi: 10.1097/ccm.0000000000003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Takenaka T, Kobori H, Miyazaki T, Suzuki H, Nishiyama A, Ishii N, Yamashita M, Hayashi M. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol (Oxf) 2019;225:e13190. doi: 10.1111/apha.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu YL, Xie J, An SW, Oliver N, Barrezueta NX, Lin MH, Birnbaumer L, Huang CL. Inhibition of TRPC6 channels ameliorates renal fibrosis and contributes to renal protection by soluble klotho. Kidney Int. 2017;91:830–841. doi: 10.1016/j.kint.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song S, Gao P, Xiao H, Xu Y, Si LY. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013;8:e82968. doi: 10.1371/journal.pone.0082968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF–FGFR–heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6:743–750. doi: 10.1016/S1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 173.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 174.Holt SG, Smith ER. Fetuin-A-containing calciprotein particles in mineral trafficking and vascular disease. Nephrol Dial Transplant. 2016;31:1583–1587. doi: 10.1093/ndt/gfw048. [DOI] [PubMed] [Google Scholar]
- 175.Pasch A, Jahnen-Dechent W, Smith ER. Phosphate, calcification in blood, and mineral stress: the physiologic blood mineral buffering system and its association with cardiovascular risk. Int J Nephrol. 2018;2018:9182078. doi: 10.1155/2018/9182078. [DOI] [PMC free article] [PubMed] [Google Scholar]