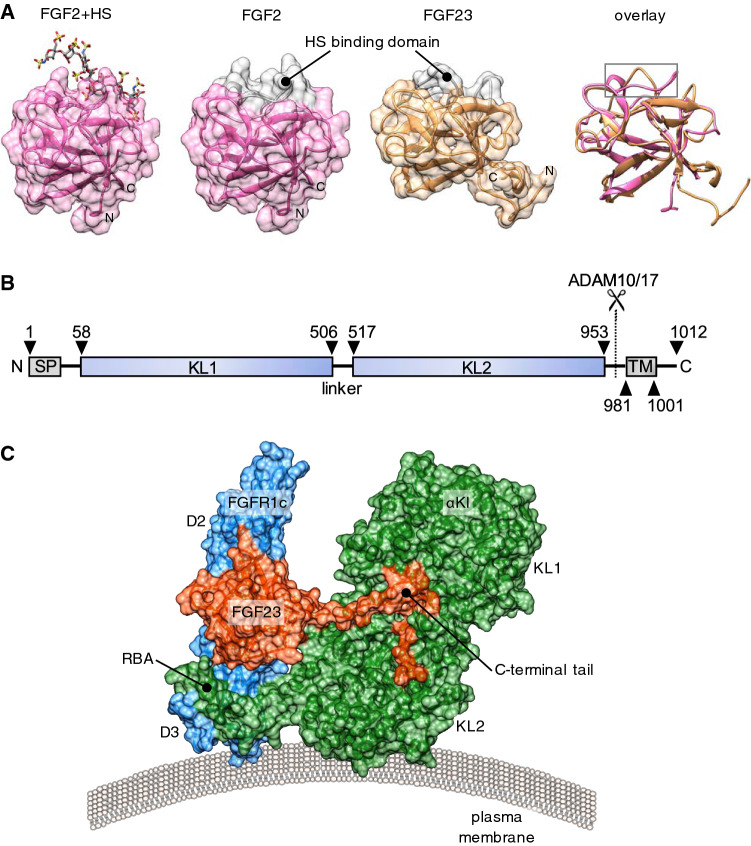
Fig. 1.
Structural basis for the endocrine action of FGF23. a From left to right: Surface representation of prototypical paracrine FGF2 with bound heparan sulphate (HS) octasaccharide (Protein Data Bank, PBD ID 1fq9) [172], aligned FGF2, FGF23 (Tyr25 to Asn170; PBD ID 2p39), and the cartoon structures overlayed [35]. Note the conserved globular core architecture but different conformations of the HS-binding (grey colour). b Cartoon depicting domain structure of 1012 amino acid human αKl. SP, TM, KL1, KL2: denotes signal peptide, transmembrane domain, tandem internal homologous KL repeats 1 and 2. The αKl ectodomain comprising KL1 and KL2 can be cleaved by plasma membrane-tethered ADAM10 or ADAM17 and released into the extracellular fluid. c Surface representation of the FGFR1c–αKl–FGF23 ternary complex from PDB ID 5w2 [48], which depicts D2 and D3 domain of human FGFR1c (blue), KL1 and KL2 domains of the ectodomain of human αKl (green) and human FGF23 (red; Tyr25 to Ser205). Note receptor binding arm (RBA) of KL2, which is necessary to stabilise the FGFR1c–αKl interaction and the tethering of the C-terminal tail of FGF23 through the KL1–KL2 cleft. Crystal structures were visualised in UCSF Chimera ver.1.13.1 [173]. Alignment was performed using the MatchMaker tool and the Needleman-Wunsch algorithm with BLOSUM-62 matrix