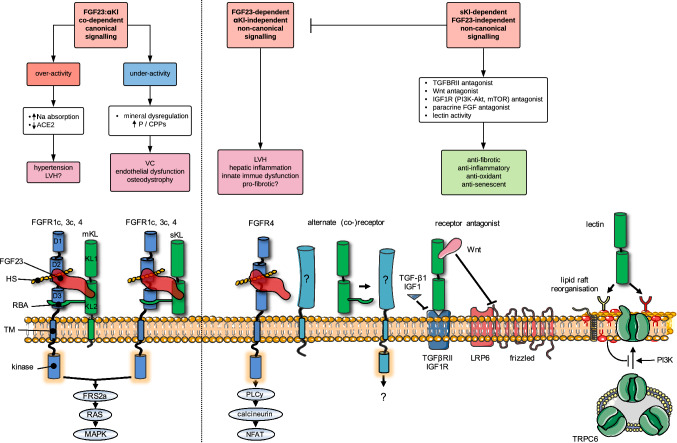
Fig. 3.
Proposed FGF23-dependent and FGF23-independent mechanisms of action of αKl and their links to pathologic signalling in CKD. Flow diagram depicts canonical (left) and non-canonical (right) pathways that may link disturbances in the FGF23:αKl axis with pathological endpoints. Molecular interactions at the cell surface that may underpin these pathways are illustrated underneath. Canonical FGF23:αKl signalling utilising either membrane-tethered αKl (mKl) or the soluble cleaved ectodomain (sKl) in complex with FGFR1c signals via the FRS2a–RAS–MAPK pathway. FGFR3c and FGFR4 may also have minor redundant roles. Over-activity of this pathway may lead to excessive sodium reabsorption, down-regulation of ACE2, hypertension and LVH. On the other hand, after loss of αKl, relative underactivity of this pathway may result in mineral dysregulation characterised by hyperphosphataemia and/or phosphate-related mineral aggregates (calciprotein particles, CPPs [174, 175]) and attendant vascular sequelae. Potentiated by the very high levels in CKD, FGF23 may also signal independently of αKl, directly driving pathology in the heart, kidney and liver. Here αKl-independent signalling is thought to occur through FGFR4 (although alternate co-receptors/co-factors may also be involved) via PLCy-calcium-dependent calcineurin-NFAT pathways. Silencing of αKl in CKD may contribute to pathological signalling through several mechanisms. Firstly, loss of soluble αKl as a decoy receptor potentiating off-target FGF23–FGFR4 or paracrine FGF signalling; secondly loss of direct protective effects of soluble αKl as a TGF-βRII or IGF1 receptor antagonist or via sequestration of Wnt proteins; thirdly, loss of homeostatic functions as a lectin, which binds α2,3-sialyllactose-containing monosialogangliosides (red lipid heads) clustered in lipid rafts and inhibits PI3K-dependent exocytosis of TRPC6. For simplicity the cartoons depict the FGF23–FGFR1c–αKl–HS in 1:1:1:1 stoichiometry rather than the 2:2:2:2 quaternary signalling complex predicted by Chen et al. [53]. D1–D3 denote immunoglobulin-like domains of FGFR. KL1 and KL2 denote tandem internal homologous KL repeats 1 and 2. ACE2, angiotensin I converting enzyme 2; Akt, serine/threonine-protein kinase; CPPs, calciprotein particles; FGF, fibroblast growth factor; FGFR, (FGF) receptor; FRF2a, fibroblast growth factor receptor substrate 2; HS, heparan sulphate; IGF1R, insulin-like growth factor 1 (IGF-1) receptor; LRP, low-density-lipoprotein-related protein; LVH, left ventricular hypertrophy; mKl, membrane αKlotho; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; Na, sodium; NFAT, nuclear factor of activated T-cells; P, phosphate; PI3K, phosphatidylinositol 3-kinases; PLCy, phospholipase C, gamma; RBA, receptor binding arm; sKl, soluble αKlotho; TGF, transforming growth factor; TGFBRII, TGF-β receptor II; TM, transmembrane domain; TRPC, transient receptor potential channel; VC, vascular calcification; Wnt, wingless integrated