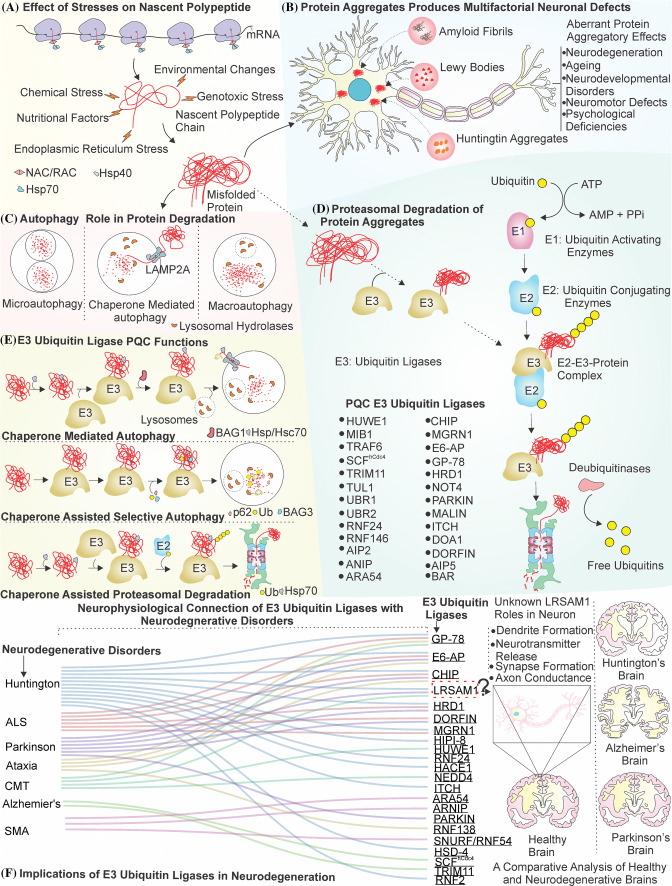
Fig. 1.
Representation of E3 ubiquitin ligases in cellular protein quality control of neurons. a Nascent polypeptide chains are under constant threat of misfolding from various stresses and are prevented from aggregation by a concerted action of various chaperones. b The misfolded polypeptides are highly prone to form aggregates in the neurons. The aggregate inclusions observed in Alzheimer’s, Parkinson and Huntington’s disease disturb the functions of neuron and can generate several kinds of protein aggregatory effects. c, d Cells remove these toxic proteinaceous inclusions by two different components of protein quality control machinery, i.e., autophagy (c) and UPS (d). e E3 ubiquitin ligases were first observed to be central in the mechanism of UPS (d) for the removal of these misfolded proteins. However, later several studies indicated that E3 ubiquitin ligases with the assistance of chaperone such as Hsp70 and other co-chaperones can communicate with both autophagy and UPS to perform the task of removing misfolded protein aggregates from the cell. f Many different types of neurodegenerative disorders are linked with a functional loss of E3 ubiquitin ligases in the cell. LRSAM1 has shown potential to act as promising E3 ubiquitin ligase with the elucidation of its functions in several neurodegenerative disorders and in neuronal physiology