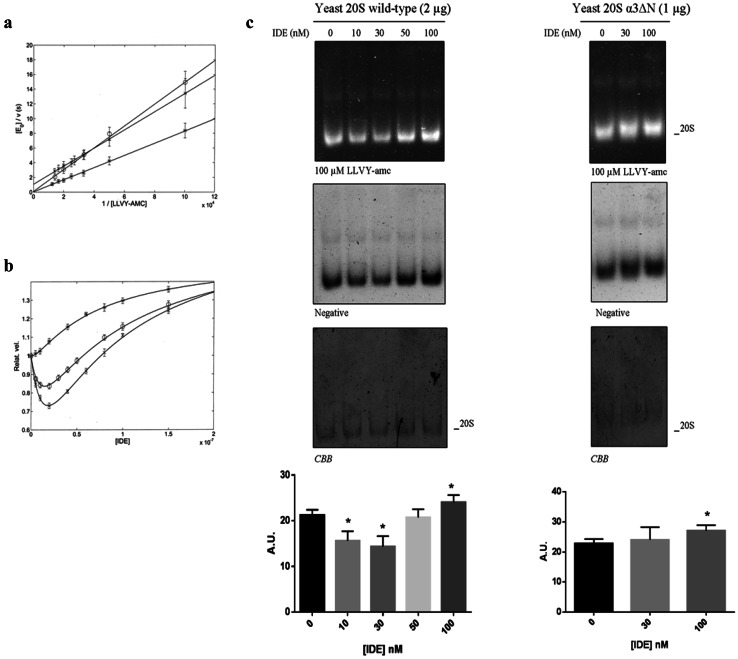
Fig. 5.
a Lineweaver–Burk plots of the substrate dependence in the absence of IDE for the chymotrypsin-like activity of humans (circle), yeast wild type (cross) and yeast α-3ΔN mutant (asterisk) at pH 7.8 and 37 °C (buffer: 25 mM Tris–HCl, pH 7.8). Continuous lines were obtained by non-linear least-squares fitting of data according to Eq. (1), employing parameters reported in Table 1. b IDE-dependent modulation of the chymotrypsin-like activity of h20S (circle), as compared to y20S proteasome [wt (cross) and α-3ΔN mutant (asterisk)] on 50 µM LLVY-amc monitored through a fluorimetric assay. Values are reported as relative activity, i.e., the activity of a specific 20S species at each IDE concentration vs that of the same 20S in the absence of IDE. c IDE effect on the wt y20S and α-3ΔN mutant activity was further examined by native gel electrophoresis. The complexes were probed with 100 µM LLVY-amc. The Coomassie Brilliant Blue (CBB) staining of the gel is shown. The relative activity of the 20S activity was quantified by densitometric analysis of the negative stain of the native gel normalized on the CBB staining for each experimental condition. Values reported are the mean ± SE of five independent experiments in the case of the wt y20S and of three independent experiments in the case of the α-3ΔN mutant. *Significantly different from the control (p < 0.001, one-way ANOVA, followed by Tukey’s test, n = 15 for wt y20S and n = 9 for α-3ΔN mutant)