Fig. 2.
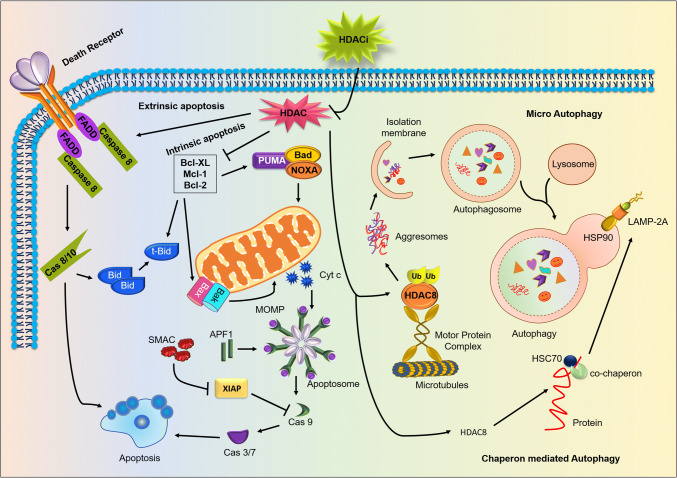
HDAC is a potent negative regulator of apoptosis and autophagy in tumorigenesis: permeabilization of HDAC inhibitor inactivates cytosolic HDAC exhibiting activation of the apoptotic and autophagic pathways, leading to negative regulation of cancer progression. HDAC inhibition mainly promotes extrinsic apoptosis by activating caspase 8 through death receptors FADD, TRADD, TRAIL, and TNF. Additionally, HDACs repress the expression of anti-apoptotic proteins like Bcl-2, Bcl-XL and Mcl-1 which in turn stimulates pro-apoptotic proteins like Bax, Bad, Bid, Bak, NOXA and PUMA. Moreover, HDAC inhibition also causes stimulation of both microautophagy and chaperone-mediated autophagy. Chaperon-mediated autophagy is initiated by the inhibition of the HDAC8 and with the help of co-chaperon HSC70. HDACs regulate the LAMP 2A deacetylation to promote autophagosome formation. Furthermore, ubiquitination of misfolded proteins is recognized by HDAC8 that results in the formation of the aggresomes and displace basal interactors of HDACs, in particular Hsp90. HDAC8 targets misfolded proteins to the microtubule-organizing center via microtubules which are degraded by the induction of microautophagy