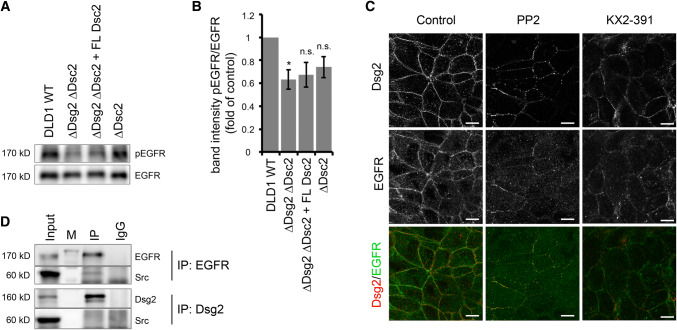
Fig. 4.
Dsg2 knockout reduces Src-dependent phosphorylation of EGFR. a Level of phosphorylated EGFR at Y845 in DLD1 cells deficient for Dsg2 and Dsc2 was analyzed via Western blot. Loss of Dsg2 resulted in reduced level of pEGFR, which was not rescued by reconstitution of Dsc2. b Intensity of bands detected by a pEGFR-specific antibody was quantified using ImageJ and normalized to total EGFR. Shown are fold-change values ± SE of six independent experiments. *p < 0.05; n.s. not significant. c Immunostaining of Dsg2 and EGFR in DLD1 cells showed reduced and fragmented staining of both proteins at the cell border after application of the Src inhibitors PP2 and KX2-391. Bar 10 µm. d DLD1 cell lysates were used for immunoprecipitation of EGFR (upper panels) or Dsg2 (lower panels) and subjected to western blot analysis for Src, revealing a co-IP of Src with EGFR but not with Dsg2