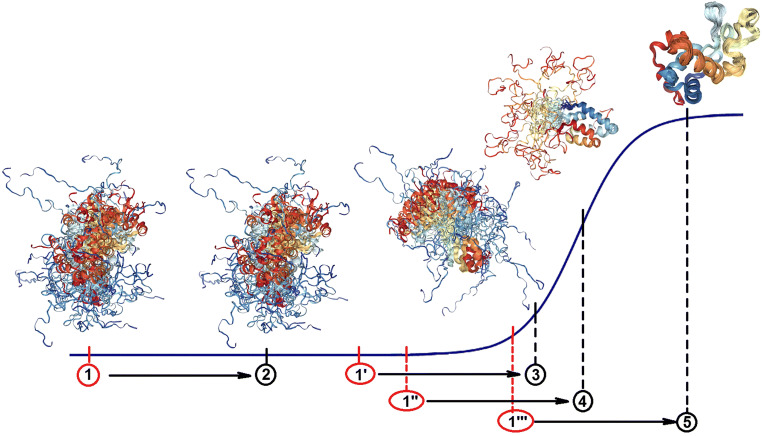
Fig. 1.
Oversimplified representation of structural transitions induced in a disordered protein by crowded environment. The overall contribution of macromolecular crowing to the total free energy of a system is the same in all four scenarios. The outputs of the presence of crowders will depend on the conformational stability of a query protein in terms of its proximity to the transition region describing structural transformation of an IDP from highly disordered to ordered state. In this model, the end result (2, 3, 4, or 5) associated with the addition of crowder to the solution of an IDP depends on a remoteness of the position of this protein from the transition region in crowder-free environment. In other words, although the distances X1 → X2, X1′ → X3, X1″ → X4, and X1′′′ → X5 are identical, they induce very different changes (ΔY1→2, ΔY1′→3, ΔY1″→4, and ΔY1′′′→5) in the conformational state of a query protein