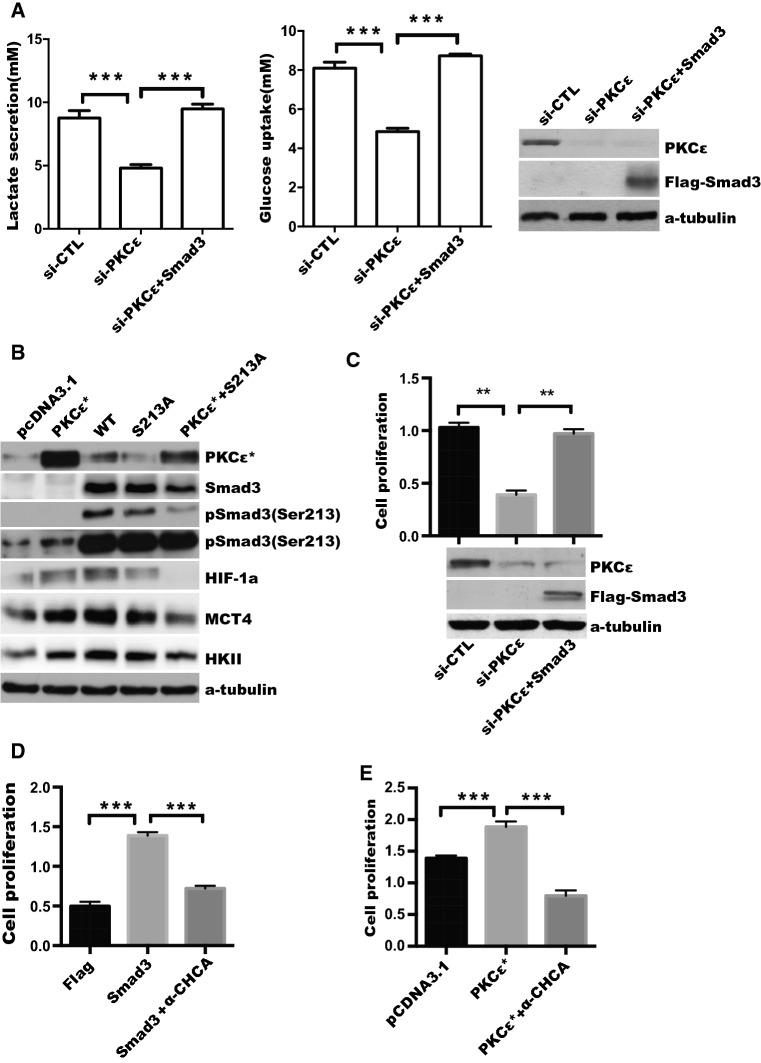
Fig. 7.
PKCε upregulates aerobic glycolysis and cell proliferation via Smad3 and lactate transporter in prostate cancer cells. a DU145 cells were co-transfected with si-PKCε and Flag-pcDNA3.1 or Flag-Smad3 (Smad3) plasmid, after 24-h transfection, cells were starved with serum-free medium for another 24 h, level of glucose and lactate production were measured as described in “Materials and methods”. Silencing of PKCε and overexpression of Smad3 were confirmed by Western blotting (right). b DU145 cells were transfected with pcDNA3.1, constitutive active mutant of PKCε (PKCε*), WT, S213A mutant of Smad3 (S213A) or PKCε* plus S213A mutant plasmids, respectively, as indicated and relative glycolytic proteins were detected by Western blotting. c DU145 cells were transfected with siCTL, si-PKCε, or si-PKCε plus Flag-Smad3, respectively. Cell proliferation was determined by CCK8 assay. Silencing of PKCε and overexpression of Smad3 were confirmed by Western blotting (bottom). d DU145 cells were transfected with Flag-pcDNA3.1 (Flag) or Flag-Smad3 (Smad3) for 18 h, and then treated with DMSO or MCT4 inhibitor (α-CHCA, 5 μg/mL) for another 24 h, the CCK-8 assay was used to measure the cell viability at OD value of 450 nm. e DU145 cells were transfected with pcDNA3.1 or the constitutively active mutant of PKCε, and then treated with the MCT4 inhibitor (α-CHCA). The CCK-8 assay as described in d. Data represent the mean ± SEM of three independent experiments and were analyzed by one-way ANOVA with multiple comparisons, followed by Dunnett’s post hoc test for significance. ***p < 0.001, **p < 0.01, and *p < 0.05