Abstract
The farnesoid-X-receptorα (FXRα; NR1H4) is one of the main bile acid (BA) receptors. During the last decades, through the use of pharmalogical approaches and transgenic mouse models, it has been demonstrated that the nuclear receptor FXRα controls numerous physiological functions such as glucose or energy metabolisms. It is also involved in the etiology or the development of several pathologies. Here, we will review the unexpected roles of FXRα on the male reproductive tract. FXRα has been demonstrated to play functions in the regulation of testicular and prostate homeostasis. Even though additional studies are needed to confirm these findings in humans, the reviewed reports open new field of research to better define the effects of bile acid-FXRα signaling pathways on fertility disorders and cancers.
Keywords: Bile acid signaling, Fertility, Cancer
Introduction
Bile acids are synthesized from cholesterol in the liver and are components of bile salts. They have major involvement in digestion. Next to this, bile acids have been defined as signaling molecules controlling many physiological functions such as lipid homeostasis, glucose and energy metabolisms. Multiple signaling pathways mobilized by bile acids (BA) ensure these functions. BAs act as ligands for several receptors among which the Farnesoid-X-Receptor-α (FXRα, NR1H4) and the G-protein-coupled bile acid receptor-1 (GPBAR1; TGR5).
The nuclear receptor of bile acids: FXRα
FXRα belongs to the family of steroid hormone receptors and is present in many species and produced by the NR1H4 gene. Four isoforms of FXRα (α1/4) have been identified resulting from alternative splicing and the use of two different promoters in the NR1H4 gene. The alternative promoters at exon 1 or exon 3 regulate the expression of FXRα1 and FXRα2 or FXRα3 and FXRα4 transcripts, respectively. The FXRα3 and FXRα4 isoforms possess longer N-terminal regions than do FXRα1 and FXRα2. The isoform differences could impact the efficiency of the “activation function 1 domain” (AF-1) for interacting with cofactors. In the FXRα1 and 3 isoforms, exon 5 is differentially spliced compared to FXRα 2 and 4. This alternative splicing event results in the addition of four amino acids (MYTG) adjacent to the DNA-binding domain in the hinge domain. It has been demonstrated that these isoforms are expressed at different levels within the tissues. Levels of FXRα1/2 are higher in the liver and adrenal glands and FXRα3/4 forms are found in the gut and kidneys [1].
FXRα is a ligand-mediated transcription factor. It was first defined as the receptor of farnesol generated by mevalonate metabolism. It was then described as the receptor of BA [2]. The most efficient ligands of FXRα are chenodeoxycholic acid (CDCA) and its conjugated forms [3]. Other BAs can activate FXRα with different degree of potency such as deoxycholic acid (DCA), lithocholic acid (LCA) and then cholic acid (CA) [2].
Over the years, multiple studies have been able to discriminate natural ligands for FXRα. Among them some act as antagonists such as guggulsterone, which was isolated from an extract of the gum resin of Commiphora mukul, and stigmasterol, which was previously used in parenteral food [4, 5]. Other molecules were defined as FXRα agonist such as cafestol, a diterpene isolated from unfiltered coffee brew [6]. It is interesting to note that bile acids have been described for long to have endocrine activity. In that line, the levels of BAs acting as endogenous FXRα agonists in vivo are essential to modulate the physiology of several organs. In fasting conditions, serum bile acid are usually below 5 µM, whereas in post-prandial the concentrations reach 15 µM [7]. The use of reporter mice showed that in basal condition, the levels of BA are sufficient to induce the activity of the FXRα receptor in different organs such as in the intestine. It has been demonstrated that in short term condition following meal, higher activity of FXRα can be observed [8].
In parallel, efforts were also put in the identification of selective and specific synthetic modulators of FXRα. Among all the agonists produced, two of them are now actively used, namely GW4064 and INT747 [9].
At the structural level, FXRα shows all the typical domains of nuclear receptors, with the dimerization interface, the ligand-binding domain, the DNA-binding domain and the ligand-dependent activation function (AF-2) domain. FXRα mainly acts as heterodimer with the retinoic acid receptor RXR (Retinoid X Receptor). The FXRα/RXR heterodimer is a permissive one, as the ligands of both partners can synergize to regulate the transcription of target genes.
The heterodimer FXRα/RXR binds to specific FXR response elements (FXRE) located in the regulatory sequences of target genes. The classical FXRE sequences correspond to hexamers (AGGTCA) that are arranged as inverted repeat motifs (IR) separated by one base (IR-1) [2]; even if other FXREs with lower affinity have been defined such as IR0, IR8, ER8 (an everted repeat motif) or DR1 (a direct repeated motif). In few cases, it has been demonstrated that FXRα can act as a monomer and thus can repress transcription through negative FXREs [1].
The use of pharmalogical approaches and of rodent transgenic models has defined the roles of FXRα receptor in the control of lipid, carbohydrate and bile acid metabolisms (for review see [10, 11]). Indeed, mouse deleted for the gene encoding FXRα have altered BA homeostasis associated with hyper-triglyceridemia and impaired glucose homeostasis. Moreover, the study of the FXRα knockout mouse models led to the identification of the involvement of FXRα signaling pathways in diseases such as diabetes, immune disorders as well as cancers. For these latters, FXRα−/− mice developed spontaneously cancerous liver lesions at 1 year old [12, 13].
The importance of FXRα in the regulation of physiological functions and the association with diseases were recently highlighted with the studies on humans and the identification of polymorphisms. FXRα variant due to single nucleotide polymorphisms, leading to reduced transcriptional activity, was identified in intrahepatic cholestasis of pregnancy (ICP) [14]. More recently another study showed that cases of neonatal cholestasis were associated with mutations in the Fxrα gene [15].
Next to these descriptions, an additional function of FXRα was added in the last years, with the identification of the involvement of BAs in the regulation of the male reproductive system [16]. Here we will review most of the data highlighting the critical functions of FXRα within testis, prostate, seminal vesicles and spermatozoa.
The idea of a link between bile acid signaling and male genital tract emerged from the association between hepatic pathologies and infertility in human [17, 18]. Indeed, during liver disorders, bile acids concentrations reached high levels. In addition, cirrhosis can be associated with testicular atrophy, low testosterone levels, decreased libido, infertility and gynaecomastia [19, 20]. Moreover, a retrospective study has correlated hepatic steatosis with low testosterone levels [21].
In the last years, different BA signaling pathways through FXRα and TGR5 have been demonstrated to be involved in male genital tract disorders [16]. As far as reproductive tract is concerned, there is no obvious reported redundancy between FXRα and TGR5. Indeed, so far, the role of TGR5 has been studied only in adult male mice using a diet enriched in cholic acid (0.5% CA), which is a classical model to mimic cholestasis. This is translated in mice by a multi-component phenotype in adequacy with the known roles of bile acids. It induces weight loss and hepatic steatosis with a decrease in hepatic Igf1 (Insulin-like growth factor-1) transcripts and altered plasma testosterone concentrations. These effects are not sufficient to explain infertility induced in cholestatic conditions since they remain present in Tgr5−/− males whose fertility and testicular physiology are preserved in response to CA-diet. In wild-type mice, CA-diet feeding leads to alteration of germ cell homeostasis relying on the control within germ cell lineage of genes involved in cell–cell interactions and thus associated with post-meiotic germ cell apoptosis [22]. This work draws attention to the major impacts of the hyper-activation of BA-signaling on the maintenance of reproductive functions in adult male mice in the context of liver disorders.
In addition, the links between BA signaling and male reproductive tract are sustained by new findings showing that testis express the genes encoding enzymes involved in BA synthesis [23]. As in the liver, FXRα controls this process. Indeed, Fxrα−/− mice showed altered BA homeostasis in both liver and testis [23]. It has appeared in the last years that FXRα plays multiple roles in the urogenital tract that are described below.
FXRα and male genital tract
FXRα and testis
Several studies demonstrate that FXRα is expressed in the human testis since the first trimester of pregnancy [24], as well as in testis of many other species such as mouse [25]. In adult mouse, the expression of FXRα was described in the interstitial space, mainly in Leydig cells [26]. This expression was confirmed at pubertal age when Leydig cells start to produce adult levels of testosterone [27]. Interestingly, the use of mouse models defines the saptio-temporal expression of FXRα within the testis. In that line, it has been demonstrated that FXRα is also expressed during post-natal development within the germ cell lineage and more particularly in undifferentiated germ cells (UGC) [28]. In addition, a study on marbled newt also showed the expression of FXRα in primordial germ cells and primary spermatogonia [29]. These localizations suggest that FXRα must be involved in the control of both the endocrine and exocrine functions of the testis.
FXRα & testicular endocrine function
The first described role of FXRα within the testis relies on its involvement on the endocrine function (Fig. 1). Indeed, the combined use of Fxrα−/− mice and of pharmacological approaches using the FXRα specific synthetic agonist GW4064 have demonstrated that the gene encoding the orphan nuclear receptor Small Heterodimer Partner (Shp; Nr0b2), a well-known FXRα target gene in the liver, is also a target gene within the Leydig cells. It was demonstrated that the increase of SHP is then associated with the repression of the expression and/or the transcriptional activity of Liver-Receptor-Homolog-1 (Lrh1; Nr5a2) and of Steroidogenic Factor-1 (Sf1; Nr5a1) receptors, two other members of the nuclear receptor superfamily, that are described to positively regulate the expression of genes involved in the steroidogenic pathways [30, 31]. These data were established using in vitro approaches such as Chromatin Immuunoprecipitation. Following the exposure to GW4064, the subsequent regulations of SHP were associated with the down-regulation of the expression of the steroidogenesis genes such as Star, Cyp11a1 and 3β-Hsd [26]. The involvement of SHP was sustained by the fact that GW4064 has no effect on testosterone levels in Shp−/− male mice. Consistently, Shp−/− mice showed higher plasma and testicular testosterone levels associated with an early maturation of the male genital tract. Indeed, male sexual maturation is a key developmental period depending on the hormonal status. The identification of the involvement of FXRα on the regulation of the testicular endocrine function led to the question of its potential involvement in male sexual maturation at puberty. This was sustained by data associating experimental models of liver disorders and altered puberty [32]. These experimental models showed lower testosterone levels and smaller testes and seminal vesicles. Recently, study deciphered the involvement of BA and FXRα in the cholestasis-induced sexual maturational failure. For that purpose, 21-days old male mice were fed a diet supplemented with cholic acid or treated with GW4064 [27]. The pubertal exposures resulted in an alteration of spermatogenesis. Indeed, the BA-exposed testis showed a lower number of elongated spermatids suggesting that the sexual maturation was delayed, which was correlated to a lower sperm production and then reduced fertility. This default in spermatogenesis was due to an increase of the apoptotic rate of germ cells. This later was related to the alteration of the hormonal homeostasis as testosterone supplementation reversed the effect of BA treatment on germ cell apoptosis.
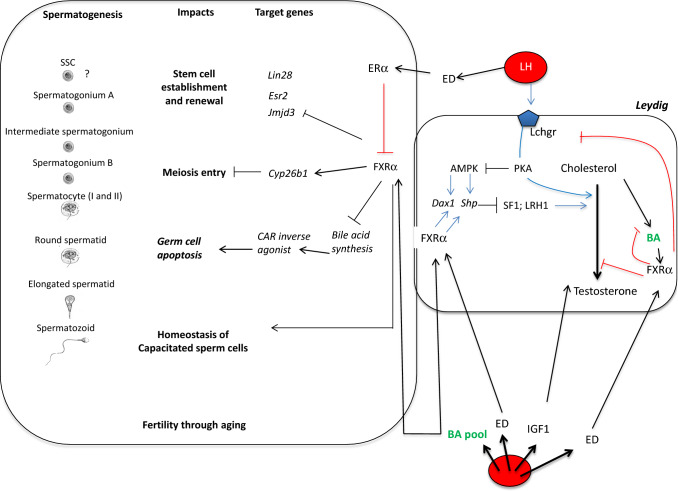
Fig. 1.
Impacts of FXRα signaling pathways within the testis. The liver secretes the insulin growth factor-1 and is involved in the control of steroid catabolism; two factors that are known to be key for testicular homeostasis. In addition, DCA produced by the microbiota is an important mediator of BA-induced testicular toxicity. IGF1, BA and LH reach the testis through blood circulation. Testicular BA pool is composed of both circulating ones and those produced within the testis. There, BA will control the Leydig endocrine function by controlling the production of steroid directly or through the decrease of the sensitivity to the LH signaling. In combination, through FXRα or TGR5, BA will control the exocrine function with impact on Sertoli cell homeostasis as well as alterations of processes involved in spermatogonial stem cell (SSC) self-renewal or germ cell survival. In addition, this later impact could also be associated with the increase of BA-precursors and the subsequent activation of CAR signaling pathways. This leads to lower sperm production and then male fertility disorders
The putative impact on testosterone synthesis was dependent on FXRα as no deleterious effects of GW4064 were observed in Fxrα−/− male mice. Unexpectedly, in contrast to adult male mice, this effect was not strictly dependent on SHP, as Shp−/− males exposed during puberty were still responsive to BA treatment [27]. It was then demonstrated that the nuclear receptor Dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 (Dax1; Nr0b1), a known repressor of steroidogenesis, was a direct target gene of FXRα. This suggests that DAX-1 might be in part responsible, in collaboration with SHP, of the negative impact of FXRα signaling pathways on steroidogenesis and thus on male sexual maturation at puberty [27].
In both adult and pubertal mice, the impacts of FXRα are local effects within the Leydig cells and independent of the hypothalamo-pituitary axis (HPA). However, some links might exist between FXRα and the hypothalamo-pituitary axis. Indeed, it was shown that FXRα also lowered testosterone synthesis through the repression of the expression of the LH receptor gene (Lhcgr). This leads to a lower sensitivity to the hypothalamo-pituitary axis, which is the major regulator of the endocrine function of the testis [27]. Moreover, it has been demonstrated that the expression of Shp and Dax-1 are down-regulated by the LH signaling [33, 34]. This suggests that local signaling pathways might participate in the precise regulation of the endocrine function in interaction with the HPA.
The involvement of FXRα on the control of testicular steroid synthesis is supported by the conserved mechanism in other species. Indeed, in porcine Leydig cells, the treatment with the FXRα agonist chenodeoxycholic acid (CDCA) decreased sex steroid production [35].
Even if exogenous BAs have been demonstrated to repress testicular steroid synthesis; the question of the endogenous testicular FXRα ligand remains. Androsterone, a metabolite of testosterone was shown to be a FXRα activator [36]. This data suggests that a local feedback loop of androgen synthesis might exist.
Moreover, next to the regulation of the androgen synthesis, some studies have demonstrated a crosstalk between FXRα signaling and the estrogen synthesis in females and males [2]. In vitro studies have demonstrated that FXRα reduced the expression of the gene encoding the aromatase [37]. At the molecular level, it was shown that FXRα competes with SF1 for binding sites on the regulatory sequences of the aromatase gene and thus its expression. However, these mechanisms still have to be elucidated in vivo.
Steroid plasma concentrations result from a balance between their synthesis and their catabolism. The liver essentially supports the inactivation and elimination of steroids. It has to be considered that the impacts of FXRα on the sexual hormones might involve steroid catabolism probably at the liver level. Indeed, in some studies it has been demonstrated that adult male mice fed a diet supplemented with 0.5% cholic acid for several months showed an altered plasma testosterone concentration with normal intra-testicular levels [22]. It was thus demonstrated that theses animals exhibited an increased hepatic expression of Cyp3a11 and Ugt2b34 suggesting hepatic catabolism of testosterone [22]. Bile acids are capable, via FXRα, to induce the expression of several catabolic enzymes such as Ugt2b4 or Cyp3a11. It can be, therefore, hypothesized that these inductions may interfere with the homeostasis of circulating steroid levels and cause a decrease in plasma testosterone concentrations.
FXRα and etiology of pubertal defect in humans?
Surprisingly, when analyzing Fxrα knock-out mice there is a decrease of intratesticular testosterone levels. This is due to the increase of SHP, a know repressor of steroidogenesis, in Leydig cells. However, this effect is transiently seen up to 1 month of age in mice. This suggests that FXRα might be involved in the control of the timing of sexual maturation. This is associated with the repressive action of Shp and Dax-1 with respect to Leydigian steroidogenesis.
Interestingly, puberty delays in boys have increased steadily in recent years [38]. The clinical definition of pubertal delay is the absence of an increase in testicular volume beyond 14 years. It is accompanied by a lack of maturation of sexual characteristics and muscle development and a lack of acceleration of the growth rate normally associated with puberty.
The most frequent diagnosis of delayed puberty (80% of cases) is a so-called “simple” pubertal delay, spontaneously correcting with time. Its etiology is often misunderstood. At the molecular level, no data reported an association between SHP or FXRα and altered puberty, In contrast, in humans, some DAX-1 mutations result in a large phenotypic variability highlighting the complexity of its functions [39]. Most of these lead to adrenal insufficiency (AHC, adrenal hypoplasia congenita) and gonadotropin deficiency associated with delayed or no puberty [40, 41].
Moreover, due to the multiple actions of FXRα for the regulation of metabolisms of bile acids, cholesterol or triglycerides, several synthetic bile acids, agonists or antagonists of FXRα, have been developed to fight against the development of pathologies such as hypertriglyceridemia [42], [43]. In this context, our study raises the question of the consequences of exposure to such molecules during puberty (pubertal delay).
FXRα and Leydig tumor?
A potential association between FXRα and Leydig tumor has also been evocated [44]. Using the R2C Leydig tumor cell line, it was demonstrated in vitro that the use of a FXRα agonist decreases the Leydig tumor growth through the inhibition of proliferation and the induction of apoptosis processes. At the molecular level, it was established that a FXRα-nuclear factor-kB (NF-kB) complex binds to the p53 promoter, inducing the expression of P53; which in turn induced its effector p21 (WAF1/Cip1).
Leydig cell tumors represent 3% of the testicular cancers and are benign in most of the cases. The above data suggest that FXRα ligands could represent a therapeutical strategy for the rare malignant Leydig tumor for which so far no treatment is available.
FXRα in male germ cells
The exocrine function of the testis corresponds to the production of spermatozoa to ensure male fertility (Fig. 1). Undifferentiated spermatogonia undergo multiple steps of transformation. Part of the spermatogonial stem cells (SSCs) self-renew and others enter in the differentiation process leading to spermatocytes. Then following the meiotic process germ cell lineage gives rise to the haploid round spermatids, which will, through spermiogenesis, become elongated spermatids and then spermatozoa.
Recent studies in mice have shown an abnormal accumulation of undifferentiated germ cells in the testes of Fxrα−/− mice [28]. It was hypothesized that FXRα helps to establish and to maintain the pool of undifferentiated germ cells and thus male fertility throughout aging. Consistently, Fxrα−/− males showed a higher production of spermatozoa compared to their wild-type littermates. This is associated with the maintenance of Fxrα−/− male fertility throughout aging at a time when wild-type fertility decline is normally observed. These data sustained the idea that FXRα participates to the homeostasis of spermatogonial stem cells (SSC), which are the only cell population to present regenerative capacity. In that line, it has been demonstrated that FXRα controls the expression of multiple genes known to be involved in the establishment of the SSC population such as Jmjd3, whose deficiency, like for Fxrα, was associated to heavier testes and maintained reproductive capacities for longer time than wild-type littermates [45]. Similarly, a closed phenotype to Fxrα−/− males was observed in the Erβ (Ers2) deficient mice with an increase in the number of UGC at postnatal age [46].
In addition, the higher expression of Lin28 following the modulation of the FXRα signaling pathways was associated with altered expression of pluritoptency genes such as Oct3/4 and Nanog highlighting the critical role of Lin28 for the development of UGC in mice [47]. Even though these data suggest that Lin28 could be a mediator of FXRα in the establishment of SSC pool, the mechanisms by which FXRα controls the expression of Lin28 remain to be identified.
This impact of FXRα on the establishment of UCG was also supported by the use of the synthetic agonist, GW4064, as it leads to a lower number of PLZF-positive seminiferous tubules [48].
Next to the impact of FXRα on the UGC pool, it also seems to participate in germ cell differentiation process. Indeed, a precocious entry in meiosis was observed in the Fxrα−/− males [28]. The crosstalk with the retinoid pathway, a major signal for germ cells to enter into meiosis, was demonstrated by the lower expression of the retinoid-degrading enzyme Cyp26b1 in Fxrα−/− male mice and the fact that a RAR (retinoic acid receptor) antagonist blocked germ cell differentiation even in Fxrα−/− males.
Combined these data suggest that FXRα might control some pathways within the germ cell lineage that permit efficient spermatogenesis in autonomous manner. Indeed, it has been demonstrated that FXRα controls germ cell survival in an androgen-independent manner in postnatal period, through the regulation of the expression of caspase-6, a known target of bile acid signaling pathways in the liver [49].
In addition, some data showed that the impacts of the lack of Fxrα gene on the sustained spermatogenesis (higher sperm cell production) were observed concomitantly to a decreased number of Sertoli cells [28], which are essential structural and nutritive support for spermatogenesis. In addition, it has been demonstrated that FXRα signaling pathways might alter the maturation of Sertoli cells during early post-natal development as a precocious alignment of Sertoli cells at the basal membrane of the seminiferous tubules was observed following the exposure to GW4064 during the 10 first days of life [48]. Even though the mechanisms were not studied, this might help to better elucidate the complex interaction between somatic Sertoli cells and germ cells differentiation.
The links between FXRα and the control of male fertility and testicular homeostasis were sustained by recent data. It has been demonstrated that mice invalidated for the gene encoding FXRα were more sensitive to BA exposure and that a short-term exposure (15 days) led to altered fertility correlated with lower sperm production due to an increased meiotic germ cell apoptosis independently of the androgen status [23]. At the molecular level, the mechanisms were in part defined and highlighted for the first time with the unexpected role of the constitutive androstane receptor (CAR, NR1I3) within the testis. Indeed, the authors have demonstrated that Fxrα−/− males fed a BA-diet showed altered testicular BA homeostasis associated with the accumulation of BA intermediate metabolites defined as negative CAR modulators. The crosstalk with CAR signaling pathways within the Fxrα−/− mice was supported by data showing that the administration of CAR agonist counteracted the impact of BA-diet [23].
Moreover, FXRα is expressed in human sperm cells mainly in the middle piece. The activation of FXRα by BA alters the sperm parameters, whereas an inhibition through the use of antagonist (Guggulsterone) or immuno-blockage inhibits these impacts. It has been demonstrated that the increased levels of BA modulate metabolic parameters (triglyceride) in sperm cells. It was thus suggested that BA could impact the homeostasis of capacitated sperm and could thus participat in infertility with idiopathic origin [50].
Fxrα, testicular spermatogenesis and environment
It has been demonstrated in liver and testis that FXRα controls the expression of many genes encoding drug transporters [51]. These data suggest that FXRα could have a major role in testicular detoxification of exogenous drugs (for review see [1]). These data led to the hypothesis that a link might exist between exogenous molecules, their deleterious impacts on male fertility and FXRα.
In that line, the increased incidence of pathologies of the male urogenital tract has been associated with the exposure to environmental molecules (EM). The list of EMs grows regularly (pollutants, pesticides, drugs) and their activity can be mediated by number of effectors. Most of these EMs, altering male reproductive functions, have been demonstrated to have anti-androgenic or estrogen-like activities [52].
Several reports have highlighted the impacts of EM exposures on bile acid metabolism [53], and several EMs have also been described to be potential FXRα modulators. Recently, additional work showed that the FXRα signaling pathway might be a critical mediator of the impacts of EMs. Indeed, it has been demonstrated that the combined exposure during fetal/neonatal periods to bisphenol-A (BPA, a well-known endocrine disruptor) and Stigmasterol (S) (natural FXRα antagonist) led to an enhanced decreased fertility compared with the exposure to single EMs [48]. This was correlated with decreased number of undifferentiated spermatogonia, of spermatids and of sperm cells, and then associated with abnormalities of the male reproductive function. The involvement of FXRα was supported by the fact that Fxrα−/− male mice were almost unaffected. It is interesting to note that BPA alone had lesser effect on the Fxrα−/− males suggesting crosstalk between FXRα and BPA signaling pathways. This was consistent with the lower accumulation of Esr2 in Fxrα−/− testis [28]. However, another explanation was given with the control of Fxrα expression by BPA signaling pathway within germ cells, similarly to what was reported in liver [54].
All these data demonstrate that FXRα acts as a major mediator of some EMs such as BPA to mediate their detrimental impacts on testicular homeostasis and thus fertility. It is important to note that these effects must be extrapolated to humans as adverse effects of the co-exposure to BPA + S were observed on the human testis in ex vivo experiments [48].
Interestingly, Hsu’s work demonstrated the antagonistic action of insecticidal xenobiotic molecules such as cyfluthrin or bifenthrin on Fxrα [55], opening a more widespread possibility of the role of FXRα in the development of environmental diseases and particularly male reproductive disorders.
Prostate/vesicle seminals: from fertility to cancer
So far the impact of the FXRα signaling pathways on the male reproductive tract were mainly studied in regards with the testicular homeostasis. However, some recent data also suggest putative impacts on seminal vesicles or prostate, two organs involved in the production of seminal plasma, which is essential to ensure the quality and integrity of sperm cells for fecundation. However, to our knowledge no data are available on the epididymis.
Recent data using proteomic analysis have identified that some proteins of the turkey seminal plasma may be involved in the regulation of lipid metabolism through FXRα activation pathways. It could thus be hypothesized that FXRα pathways must be important for sperm after ejaculation [56]. This could be consistent with the expression of FXRα in spermatozoa as demonstrated in human [50].
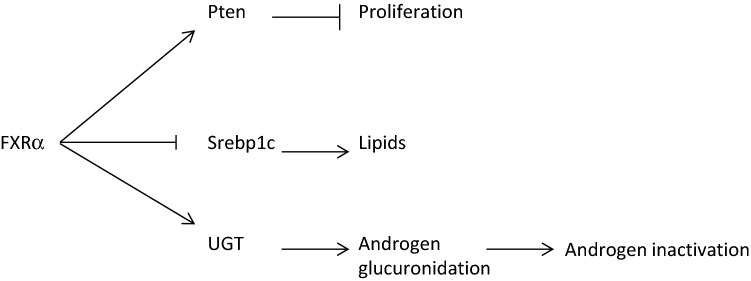
Within the prostate, the role of FXRα was mainly studied in the context of tumor cells (Fig. 2). It has been demonstrated that the mRNA and protein accumulation of FXRα are decreased in prostate cancer biopsies [57]. In vitro approaches have demonstrated that FXRα activation increased the expression of the tumor suppressor PTEN and thus suppressed prostate cancer cell proliferation [57]. This later effect on cell growth was only observed in tumoral [58]. In addition, it has been demonstrated, consistently with known roles of FXRα in other organs, that its activation in prostate lowered lipid accumulation through the regulation of sterol regulatory element-binding protein 1 (SREBP1).
Fig. 2.
Impacts of FXRα signaling pathways on prostate
Interestingly, it was shown that FXRα activation represses the expression of UDP-glucuronosyltransferase (UGT) 2B15 and UGT2B17 within prostate cancer LNCaP cells. As glucuronidation inactivated androgens it must be hypothesized that FXRα signaling pathways will control androgen metabolism in prostate cancer cells. This must be critical since androgens are major regulators of prostate cell growth and physiology [59].
Bile acid signaling, metabolic syndrome and male reproductive tract
It is now well established that the metabolic syndrome (MetS) is associated with defects of male reproductive tract and functions. Indeed, obesity has been demonstrated to increase the risk of reproductive disorders with lower number of motile spermatozoa [60, 61]. In addition, the prevalence of infertility in diabetic men is higher than in normal population [62]. Patients show a lower percentage of motile sperm cells, a higher number of sperm cells with altered mitochondrial function [63] and an increase of DNA fragmentation in spermatozoa [64, 65]. Metabolic syndrome (MetS) is also associated with an increased risk of benign prostatic hyperplasia and lower urinary tract symptoms (LUTS). It has been demonstrated that worse oncologic issues of prostate cancer were associated to MetS. A recently established rabbit model of high-fat diet (HFD)-induced MetS showed hypogonadism and the presence of prostate gland alterations, including inflammation [66].
The possibility to target BA pathways for the cure of metabolic diseases is promising. Indeed, it is associated in experimental models to the improvement of metabolic syndrome parameters (cholesterol, triglycerides an glucose levels). Moreover, in the model of HFD-induced MetS in rabbit, it has been demonstrated that treatment with the FXRα agonist (INT747) counteracted the impact on bladder suggesting that is might be a consequence of MetS improvement [50]. However, INT-747 did not revert the MetS-induced hypogonadal state [67]. Consistently, the data suggest that during treatment of MetS through the use of BA-enriched diet, male mice fertility was additively affected by HFD and BA-die whereas parameters such as cholesterol, triglycerides and glucose levels were improved [68]. Further studies are thus needed to overcome the negative impacts of BA-signaling pathways before using them safely for the treatment of chronic diseases related to MetS.
Conclusions and perspectives
The reported data highlight the emerging roles of the BA-FXRα signaling pathways in the control of the reproductive tract physiology.
Adult mice fed a diet supplemented with cholic acid showed increases in both plasma and intra-testicular bile acid concentrations associated with subfertility of 25% of the exposed males. It could be expected that this might rely on complex integrated signaling through the whole male physiology. As several organs have been demonstrated to control the physiology of the testis (see Figs. 1 and 3), this raises the question of the origin of the multiple alterations, which could be either direct impacts on the gonads or indirect effects resulting from systemic disorders.
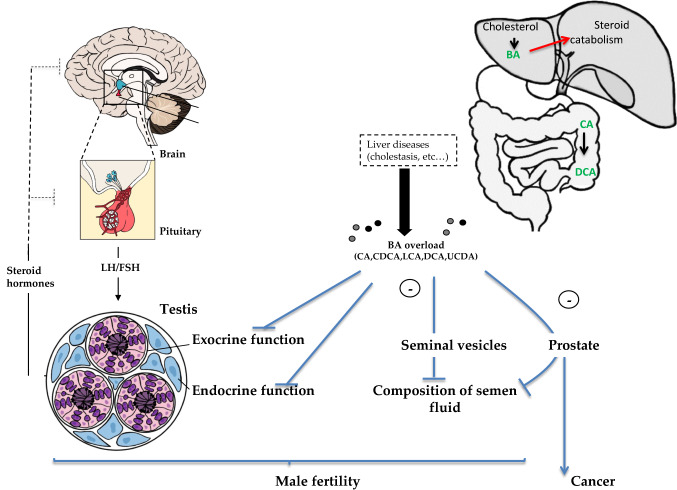
Fig. 3.
Integrative model. The impact of liver disorders leading to male fertility disorders and particularly testicular physiology alterations rely on complex integrated signaling through the whole male physiology. The BA will alter the physiology of testis, seminal vesicle and prostate. Combined these effects might participate in the altered fertility observed during liver disorders. BA increase could also be involved in the etiology of prostate cancer
The liver has been demonstrated to secrete the insulin growth factor-1 and to be involved in the control of testosterone catabolism; two factors that are known to be key for testicular homeostasis. However, recent data suggest that in the context of cholestasis induced by supplementation of bile acids, these parameters do not seem to be essential to drive the deleterious impacts on testicular physiopathology.
It is interesting to note that both plasma and intra-testicular levels of DCA correlate with fertility disorder suggesting that the intestinal microbiota plays a major role in the testicular pathophysiology.
Even if it cannot be excluded that systemic factors are involved in BA-induced testicular disorders in wild mice, they might create a favorable context leading to the increase of bile acid levels and their deleterious impacts within the testis; as sustained by in vitro data on an isolated germ cell line (GC1spg) or Leydig cell line (primary culture and MA10 cell line).
The direct role within the testis is also supported by data showing that in the context of cholestasis, there is a decrease in testosterone synthesis associated with an increase of LH concentrations. It thus demonstrated that it is a primary hypogonadism that must find its origin in testis cells.
In addition, the testicular phenotype of Fxrα knockout on testis might not be a secondary effect induced by a disturbed liver function (e.g. via endocrine disruptors or altered hepatic clearance rates). Indeed, if endocrine disruptors or liver disorders are deleterious for male reproductive function, recent works demonstrate that Fxrα−/− males have an efficient reproductive function with the maintenance of fertility through aging compared to wild-type males.
A remaining question is to characterize the physiological ligand(s) of FXRα within the male genital tract. Data showed detectable levels and production of BA within the testis. The question remains whether BA comes from a local synthesis or that they come from the peripheral synthesis (liver and intestine). The Leydig cells, in particular, are the source of a testicular synthesis of bile acids, which is logical since, as steroidogenic cells, they have a large reserve of cholesterol. Within the testis, it could be of interest to measure local BA concentrations in specific cell types to ensure in which kind of cell types, BA could activate their signaling pathways.
In addition, previous reports demonstrate that steroids such as androsterone could be physiological ligands of FXRα and be active at least in vitro to control prostate cell homeostasis.
Moreover, BA receptors could also be modulated by other kinds of molecules. In that line, among the presented data, some of them highlight a new link between FXRα and the increasing question of the environmental origin of diseases. Indeed it must be of interest to better define if FXRα could interfere with the signaling pathways of EMs exhibiting estrogenic properties. Moreover, it will be of interest to screen EMs to define if some of them can act as exogenous ligands of FXRα, and thus define, depending on the age at exposure, whether it will impact the exocrine and/or the exocrine functions of the testis, as well as the development of prostate cancer.
The overall data presented here highlight the necessity to decipher the roles of FXRα in urogenital tract in normal and pathological conditions to define novel approaches to improve male fertility capacities, in particular in the context of environmental exposures. One of the remaining questions is to decipher the precise activity of the BA receptor in the etiology of the urogenital disorders such as puberty delay, as presented above. Extended work on FXRα will also help to define whether it could be defined as targetable pathway for the treatments of pathologies such as fertility disorders and/or cancers.
Funding
The study was funded by Inserm, CNRS, Université Clermont Auvergne, Région Auvergne (#R12087CC to DHV), Plan cancer (C14012CS), Ligue contre le cancer (comité Puy de Dôme), ARC (R16142CC). Volle’s team received support from the French government IDEX-ISITE initiative 16-IDEX-0001 (CAP 20-25).
Compliance with ethical standards
Conflict of interest
The authors declare to have no conflict of interests.
Footnotes
Manon Garcia and Laura Thirouard participates equally to this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Claude Beaudoin, Email: claude.beaudoin@uca.fr.
David H. Volle, Email: david.volle@inserm.fr
References
- 1.Garcia M, Thirouard L, Sedès L, et al. Nuclear receptor metabolism of bile acids and xenobiotics: a coordinated detoxification system with impact on health and diseases. Int J Mol Sci. 2018 doi: 10.3390/ijms19113630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baptissart M, Vega A, Martinot E, et al. Farnesoid X receptor alpha: a molecular link between bile acids and steroid signaling? Cell Mol Life Sci CMLS. 2013;70:4511–4526. doi: 10.1007/s00018-013-1387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baptissart M, Vega A, Maqdasy S, et al. Bile acids: from digestion to cancers. Biochimie. 2012 doi: 10.1016/j.biochi.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Carter BA, Taylor OA, Prendergast DR, et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–306. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 5.Owsley E, Chiang JYL. Guggulsterone antagonizes farnesoid X receptor induction of bile salt export pump but activates pregnane X receptor to inhibit cholesterol 7alpha-hydroxylase gene. Biochem Biophys Res Commun. 2003;304:191–195. doi: 10.1016/S0006-291X(03)00551-5. [DOI] [PubMed] [Google Scholar]
- 6.Ricketts M-L, Boekschoten MV, Kreeft AJ, et al. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- 7.Everson GT. Steady-state kinetics of serum bile acids in healthy human subjects: single and dual isotope techniques using stable isotopes and mass spectrometry. J Lipid Res. 1987;28:238–252. [PubMed] [Google Scholar]
- 8.Houten SM, Volle DH, Cummins CL, et al. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007;21:1312–1323. doi: 10.1210/me.2007-0113. [DOI] [PubMed] [Google Scholar]
- 9.Maloney PR, Parks DJ, Haffner CD, et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 10.Teodoro JS, Rolo AP, Palmeira CM. Hepatic FXR: key regulator of whole-body energy metabolism. Trends Endocrinol Metab. 2011;22:458–466. doi: 10.1016/j.tem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, He Q, Wang G, et al. FXR modulators for enterohepatic and metabolic diseases. Expert Opin Ther Pat. 2018;28:765–782. doi: 10.1080/13543776.2018.1527906. [DOI] [PubMed] [Google Scholar]
- 12.Kim I, Morimura K, Shah Y, et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, Huang X, Yi T, et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 14.Van Mil SWC, Milona A, Dixon PH, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Ospina N, Potter CJ, Xiao R, et al. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun. 2016;7:10713. doi: 10.1038/ncomms10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sèdes L, Martinot E, Baptissart M, et al. Bile acids and male fertility: from mouse to human? Mol Aspects Med. 2017 doi: 10.1016/j.mam.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Durazzo M, Premoli A, Di Bisceglie C, et al. Alterations of seminal and hormonal parameters: an extrahepatic manifestation of HCV infection? World J Gastroenterol. 2006;12:3073–3076. doi: 10.3748/wjg.v12.i19.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mooradian AD, Shamma’a M, Salti I, Cortas N. Hypophyseal-gonadal dysfunction in men with non-alcoholic liver cirrhosis. Andrologia. 1985;17:72–79. doi: 10.1111/j.1439-0272.1985.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 19.Burra P. Liver abnormalities and endocrine diseases. Best Pract Res Clin Gastroenterol. 2013;27:553–563. doi: 10.1016/j.bpg.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Karagiannis A, Harsoulis F. Gonadal dysfunction in systemic diseases. Eur J Endocrinol. 2005;152:501–513. doi: 10.1530/eje.1.01886. [DOI] [PubMed] [Google Scholar]
- 21.Völzke H, Aumann N, Krebs A, et al. Hepatic steatosis is associated with low serum testosterone and high serum DHEAS levels in men. Int J Androl. 2010;33:45–53. doi: 10.1111/j.1365-2605.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- 22.Baptissart M, Vega A, Martinot E, et al. Bile acids alter male fertility through G-protein-coupled bile acid receptor 1 signaling pathways in mice. Hepatology. 2014;60:1054–1065. doi: 10.1002/hep.27204. [DOI] [PubMed] [Google Scholar]
- 23.Martinot E, Baptissart M, Vega A, et al. Bile acid homeostasis controls CAR signaling pathways in mouse testis through FXRalpha. Sci Rep. 2017;7:42182. doi: 10.1038/srep42182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muczynski V, Lecureuil C, Messiaen S, et al. Cellular and molecular effect of MEHP involving LXRα in human fetal testis and ovary. PLoS One. 2012;7:e48266. doi: 10.1371/journal.pone.0048266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maqdasy S, Baptissart M, Vega A, et al. Cholesterol and male fertility: what about orphans and adopted? Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Volle DH, Duggavathi R, Magnier BC, et al. The small heterodimer partner is a gonadal gatekeeper of sexual maturation in male mice. Genes Dev. 2007;21:303–315. doi: 10.1101/gad.409307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baptissart M, Martinot E, Vega A, et al. Bile acid-FXRα pathways regulate male sexual maturation in mice. Oncotarget. 2016 doi: 10.18632/oncotarget.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinot E, Sèdes L, Baptissart M, et al. The bile acid nuclear receptor FXRα is a critical regulator of mouse germ cell fate. Stem Cell Rep. 2017;9:315–328. doi: 10.1016/j.stemcr.2017.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfaro JM, Ricote M, Lobo MVT, et al. Immunohistochemical detection of the retinoid acid receptors (RXR-alpha, -beta, -gamma) and Farnesoid X-activated receptor (FXR) in the marbled newt along the annual cycle. Mol Reprod Dev. 2002;62:216–222. doi: 10.1002/mrd.10104. [DOI] [PubMed] [Google Scholar]
- 30.Bakke M, Zhao L, Hanley NA, Parker KL. SF-1: a critical mediator of steroidogenesis. Mol Cell Endocrinol. 2001;171:5–7. doi: 10.1016/S0303-7207(00)00384-1. [DOI] [PubMed] [Google Scholar]
- 31.Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250–260. doi: 10.1016/j.tcb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Van Thiel DH, Gavaler JS, Zajko AB, Cobb CF. Consequences of complete bile-duct ligation on the pubertal process in the male rat. J Pediatr Gastroenterol Nutr. 1985;4:616–621. doi: 10.1097/00005176-198508000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Vega A, Martinot E, Baptissart M, De Haze A, Saru JP, Baron S, Caira F, Schoonjans K, Lobaccaro JM, Volle DH. Identification of the linkbetween the hypothalamo-pituitary axis and the testicular orphan nuclear receptor NR0B2 in adult male mice. Endocrinology. 2014;156(2):660–669. doi: 10.1210/en.2014-1418. [DOI] [PubMed] [Google Scholar]
- 34.Ahn SW, Gang G-T, Kim YD, et al. Insulin directly regulates steroidogenesis via induction of the orphan nuclear receptor DAX-1 in testicular Leydig cells. J Biol Chem. 2013;288:15937–15946. doi: 10.1074/jbc.M113.451773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray MA, Squires EJ. Effects of nuclear receptor transactivation on steroid hormone synthesis and gene expression in porcine Leydig cells. J Steroid Biochem Mol Biol. 2013;133:93–100. doi: 10.1016/j.jsbmb.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Lai K, Moy FJ, et al. The nuclear hormone receptor farnesoid X receptor (FXR) Is activated by androsterone. Endocrinology. 2006;147:4025–4033. doi: 10.1210/en.2005-1485. [DOI] [PubMed] [Google Scholar]
- 37.Catalano S, Malivindi R, Giordano C, et al. Farnesoid X receptor, through the binding with steroidogenic factor 1-responsive element, inhibits aromatase expression in tumor leydig cells. J Biol Chem. 2010;285:5581–5593. doi: 10.1074/jbc.M109.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoeters G, Den Hond E, Dhooge W, et al. Endocrine disruptors and abnormalities of pubertal development. Basic Clin Pharmacol Toxicol. 2008;102:168–175. doi: 10.1111/j.1742-7843.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 39.Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185:17–25. doi: 10.1016/S0303-7207(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 40.Seminara SB, Achermann JC, Genel M, et al. X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. J Clin Endocrinol Metab. 1999;84:4501–4509. doi: 10.1210/jcem.84.12.6172. [DOI] [PubMed] [Google Scholar]
- 41.Tabarin A, Achermann JC, Recan D, et al. A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest. 2000;105:321–328. doi: 10.1172/JCI7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas C, Pellicciari R, Pruzanski M, et al. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 43.Manti S, Romano C, Chirico V, et al. Nonalcoholic Fatty liver disease/non-alcoholic steatohepatitis in childhood: endocrine-metabolic “mal-programming”. Hepat Mon. 2014;14:e17641. doi: 10.5812/hepatmon.17641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catalano S, Panza S, Malivindi R, et al. Inhibition of Leydig tumor growth by farnesoid X receptor activation: the in vitro and in vivo basis for a novel therapeutic strategy. Int J Cancer. 2013;132:2237–2247. doi: 10.1002/ijc.27915. [DOI] [PubMed] [Google Scholar]
- 45.Iwamori N, Iwamori T, Matzuk MM. H3K27 demethylase, JMJD3, regulates fragmentation of spermatogonial cysts. PLoS One. 2013;8:e72689. doi: 10.1371/journal.pone.0072689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delbès G, Levacher C, Pairault C, et al. Estrogen receptor beta-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology. 2004;145:3395–3403. doi: 10.1210/en.2003-1479. [DOI] [PubMed] [Google Scholar]
- 47.West JA, Viswanathan SR, Yabuuchi A, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sèdes L, Desdoits-Lethimonier C, Rouaisnel B, et al. Crosstalk between BPA and FXRα signaling pathways lead to alterations of undifferentiated germ cell homeostasis and male fertility disorders. Stem Cell Rep. 2018 doi: 10.1016/j.stemcr.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rust C, Wild N, Bernt C, et al. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem. 2009;284:2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
- 50.Malivindi R, Santoro M, De Rose D, et al. Activated-farnesoid X receptor (FXR) expressed in human sperm alters its fertilising ability. Reprod Camb Engl. 2018;156:249–259. doi: 10.1530/REP-18-0203. [DOI] [PubMed] [Google Scholar]
- 51.Maeda T, Miyata M, Yotsumoto T, et al. Regulation of drug transporters by the farnesoid X receptor in mice. Mol Pharm. 2004;1:281–289. doi: 10.1021/mp0499656. [DOI] [PubMed] [Google Scholar]
- 52.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 53.Vega A, Baptissart M, Martinot E, et al. Hepatotoxicity induced by neonatal exposure to diethylstilbestrol is maintained throughout adulthood via the nuclear receptor SHP. Expert Opin Ther Targets. 2014;18:1367–1376. doi: 10.1517/14728222.2014.964209. [DOI] [PubMed] [Google Scholar]
- 54.Susiarjo M, Xin F, Stefaniak M, et al. Bile acids and tryptophan metabolism are novel pathways involved in metabolic abnormalities in BPA-exposed pregnant mice and male offspring. Endocrinology. 2017 doi: 10.1210/en.2017-00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu C-W, Zhao J, Huang R, et al. Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Sci Rep. 2014;4:6437. doi: 10.1038/srep06437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slowinska M, Nynca J, Arnold GJ, et al. Proteomic identification of turkey (Meleagris gallopavo) seminal plasma proteins. Poult Sci. 2017;96:3422–3435. doi: 10.3382/ps/pex132. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Tong S-J, Wang X, Qu L-X. Farnesoid X receptor inhibits LNcaP cell proliferation via the upregulation of PTEN. Exp Ther Med. 2014;8:1209–1212. doi: 10.3892/etm.2014.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu N, Zhao J, Wang J, et al. Farnesoid X receptor ligand CDCA suppresses human prostate cancer cells growth by inhibiting lipid metabolism via targeting sterol response element binding protein 1. Am J Transl Res. 2016;8:5118–5124. [PMC free article] [PubMed] [Google Scholar]
- 59.Kaeding J, Bouchaert E, Bélanger J, et al. Activators of the farnesoid X receptor negatively regulate androgen glucuronidation in human prostate cancer LNCAP cells. Biochem J. 2008;410:245–253. doi: 10.1042/BJ20071136. [DOI] [PubMed] [Google Scholar]
- 60.Hammoud AO, Gibson M, Peterson CM, et al. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 61.Hofny ERM, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril. 2010;94:581–584. doi: 10.1016/j.fertnstert.2009.03.085. [DOI] [PubMed] [Google Scholar]
- 62.Wiebe JC, Santana A, Medina-Rodríguez N, et al. Fertility is reduced in women and in men with type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium (T1DGC) Diabetologia. 2014;57:2501–2504. doi: 10.1007/s00125-014-3376-8. [DOI] [PubMed] [Google Scholar]
- 63.La Vignera S, Condorelli RA, Di Mauro M, et al. Reproductive function in male patients with type 1 diabetes mellitus. Andrology. 2015;3:1082–1087. doi: 10.1111/andr.12097. [DOI] [PubMed] [Google Scholar]
- 64.Agbaje IM, McVicar CM, Schock BC, et al. Increased concentrations of the oxidative DNA adduct 7,8-dihydro-8-oxo-2-deoxyguanosine in the germ-line of men with type 1 diabetes. Reprod Biomed Online. 2008;16:401–409. doi: 10.1016/S1472-6483(10)60602-5. [DOI] [PubMed] [Google Scholar]
- 65.Barták V, Josífko M, Horácková M. Juvenile diabetes and human sperm quality. Int J Fertil. 1975;20:30–32. [PubMed] [Google Scholar]
- 66.Morelli A, Comeglio P, Filippi S, et al. Testosterone and farnesoid X receptor agonist INT-747 counteract high fat diet-induced bladder alterations in a rabbit model of metabolic syndrome. J Steroid Biochem Mol Biol. 2012;132:80–92. doi: 10.1016/j.jsbmb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Maneschi E, Morelli A, Filippi S, et al. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol. 2012;215:347–362. doi: 10.1530/JOE-12-0333. [DOI] [PubMed] [Google Scholar]
- 68.Vega A, Martinot E, Baptissart M, et al. Bile acid alters male mouse fertility in metabolic syndrome context. PLoS One. 2015;10:e0139946. doi: 10.1371/journal.pone.0139946. [DOI] [PMC free article] [PubMed] [Google Scholar]