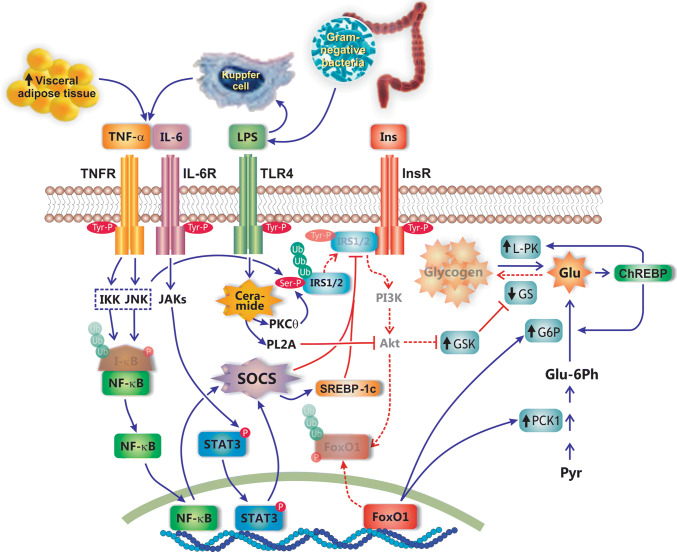
Fig. 5.
Mechanisms of hepatic insulin resistance in NAFLD. This pathology occurs in a context of sub-acute inflammation (e.g., obesity, type 2 mellitus diabetes), which promotes insulin resistance via the influx of pro-inflammatory cytokines, such as TNF-α and IL-6, from both the adipose tissue and Kupffer cells. After interaction with their respective membrane receptors, TNFR and IL-6R, these cytokines activate different signaling pathways that result in ubiquitin-mediated proteosomal degradation of the insulin receptor substrates 1 and 2 (IRS1/2), two signaling molecules required for insulin transduction via the phosphoinositide 3-kinase (PI3K)/Akt pathway. TNF-α and IL-6 promote IRS1/2 degradation by inducing synthesis of suppressor of cytokine signaling (SOCS), via activation of the transcription factors NF-κB and the signal transducer and activator of transcription 3 (STAT3), respectively. NF-κB is activated by TNF-α via inhibitor of κB (I-κB) kinase (IKK)- and c-Jun N-terminal kinase (JNK)-mediated phosphorylation, and further proteosomal degradation of the NF-κB inhibitor I-κB. IL-6 activates STAT3 via phosphorylation of this transcription factor by Janus activated kinase (JAK). Alternatively, proteosomal degradation of IRS1/2 is promoted by lipopolysaccharide (LPS) from intestinal microbiota. LPS interacts with the toll-like receptor (TLR4) to activate the ceramide/PKCθ signaling pathway, which induces, in turn, serine phosphorylation of IRS1/2 and further proteosomal degradation; ceramide also activates phospholipase-A2 (PLA2), which inhibits Akt activation. Lack of Akt signaling impairs Akt-mediated phosphorylation and further ubiquitin-mediated proteosomal degradation of forkhead box protein O1 (FoxO1), a transcription factor whose activation induces upregulation of gluconeogenic enzymes, such as phosphoenolpyruvate carboxykinase (PCK1) and glucokinase-6 phosphatase (G6P). The resulting increase in hepatocellular glucose (Glu) levels stimulates the transcription factor carbohydrate response element-binding protein (ChREBP), which in turn reinforces G6P activation and induces upregulation of enzymes involved in glycogenolysis, such as liver pyruvate kinase (L-PK), thus resulting in a higher hepatocellular glucose increase. Glycogenolysis is exacerbated by lack of Akt-mediated glycogen synthase kinase (GSK), an enzyme that inhibits glycogen synthase (GS). Blue, continuous lines: activation pathways. Red, continuous lines: inhibitory pathways. Red, dashed lines: inactivated pathways