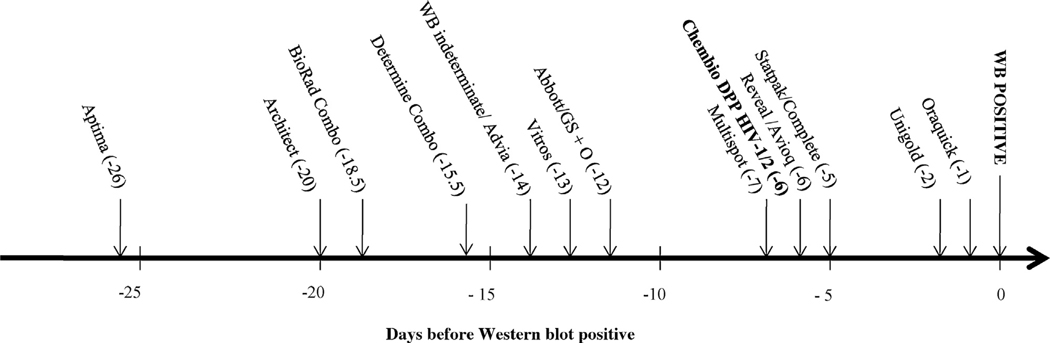
Fig. 1.
Sensitivity of assay reactivity during early HIV-1 infections as number of days before first positive WB when 50% of specimens tested with each test became positive. The names, abbreviations, and sources, of the HIV assays previously evaluated 11,12 are as follows: APTIMA HIV-1 Quantitative assay (Aptima, Gen-Probe, Inc., San Diego, CA); ARCHITECT® HIV Ag/Ab Combo assay (Architect; Abbott Diagnostics, Wiesbaden Germany; CE marked version was used as the US version was not available when testing was conducted); GS HIV Combo Ag/Ab (BioRad Combo; Bio-Rad Laboratories, Redmond, WA); DetermineTM HIV-1/2 Ag/Ab Combo (Determine Combo (rapid test); Alere Medical Co., Ltd. Scarborough, ME); GS HIV-1/HIV-2 PLUS O EIA (GS + O; Bio-Rad Laboratories, Redmond, WA); VITROS anti-HIV 1 + 2 assay (Vitros; Ortho-Clinical Diagnostics, Buckinghamshire, UK); ADVIA Centaur HIV 1/O/2 enhanced assay (Advia; Bayer, Tarrytown, NY); Abbott HIVAB HIV-1/2 (rDNA) EIA (Abbott; Abbott Laboratories, Abbott Park, IL); Avioq HIV-1 Microelisa system (Avioq; Avioq, Inc, Rockville, MD); Multispot HIV-1/HIV-2 rapid test (Multispot (rapid test); Bio-Rad Laboratories); Clearview HIV-1/2 STAT-PAK (Statpak (rapid test); Inverness Medical, Princeton, NJ); Clearview Complete HIV-1/2 (Complete (rapid test); Inverness Medical); Reveal G2 and G3 Rapid HIV-1 antibody tests (Reveal G2 or G3 (rapid test); MedMira Laboratories, Inc.; Halifax, Nova Scotia, Canada); OraQuick ADVANCE Rapid HIV-1/2 antibody test (Oraquick (rapid test); OraSure Technologies, Inc.; Bethlehem, PA); Uni-Gold Recombigen HIV (Unigold (rapid test); Trinity Biotech USA, St. Louis, MO). These assays have manufacturer reported point estimates for sensitivity ranging from 99.60% to 100.00% and point estimates for specificity ranging from 98.60% to 99.90%. The Genetic Systems HIV-1 Western blot (WB; Bio-Rad Laboratories) and Cambridge Biotech HIV-1 Western blot (WB; Maxim Biomedical Inc., Rockville, MD) have been shown to give concordant interpretations in studies conducted to qualify use in our clinical laboratory and were used interchangeably.