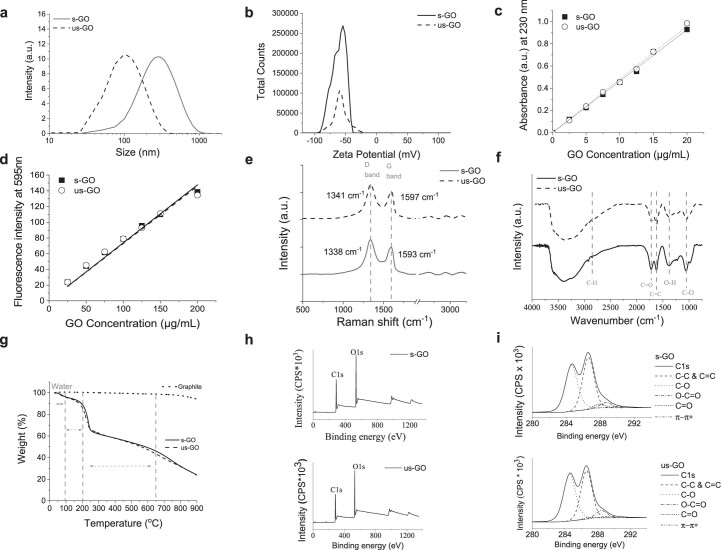
Extended Data Fig. 1. Physicochemical properties of s-GO and us-GO.
a. Dynamic light scattering (DLS) confirms the size reduction from s-GO to us-GO; b. Electrophoretic light scattering (ELS) shows highly negatively charged particles, suggesting a high colloidal stability; c. Absorption spectroscopy confirms the C=C grapheneous backbone signature through its absorbance linearity at 230 nm; d. Upon excitation with λexc = 525 nm (fluorescence spectroscopy), a maximum band at λem ~ 595 nm is generated by electrons transitioning between the oxidized and non-oxidized carbon atoms of the GO sheets; e. Raman spectroscopy highlights a GO-specific spectral signature through D and G bands, an indicator of functional groups (defects) presence onto the grapheneous backbone; f. Fourier-transform infrared spectroscopy (FT-IR) confirms the presence of functional groups such as OH, CH, C=O; C=C and C-O; g. Thermal gravimetric analysis (TGA) also confirms the oxygenated nature of s-GO and us-GO, which show particular degradation steps that are associated with the disruption of COOH, C=O, OH, C-O-C, as opposed to graphite which is stable at temperatures below 600 °C; h. X-Ray photoelectron spectroscopy (XPS) for the survey spectra indicate highly-pure materials of rich oxygen content. i. X-Ray photoelectron spectroscopy (XPS) for the high-resolution C1s spectra mainly involved in C-O, C=O and O-C-O covalent bonds. a.u. = arbitrary units; CPS = counts per second; eV = electronvolt. Data are presented as mean values ± SEM. n-numbers. Representative data of the technical replicates performed are shown.