Fig. (2).
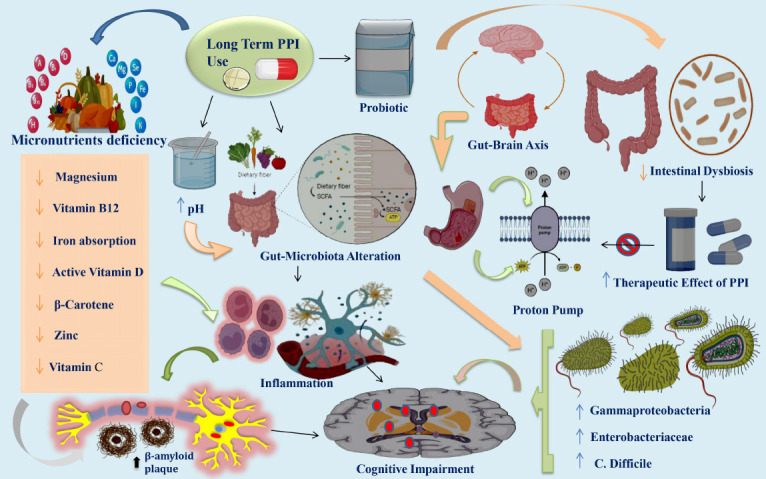
PPI-induced micronutrient deficiencies, gut-brain axis, and cognitive implications: an integrated perspective. The provided figure illustrates that extended use of PPIs is linked to adverse outcomes despite being widely recognized for their effectiveness and tolerability. Notably, these include reduced levels of essential micronutrients, such as magnesium, vitamin B12 deficiency, calcium and iron absorption, β-carotene, active vitamin D, zinc, and vitamin C [57-59]. A correlation exists between insufficiencies of these micronutrients and cognitive dysfunction and the development of Aβ plaques [55]. The gut-brain axis supports bidirectional communication between the enteric and central nervous systems by connecting the peripheral functions of the intestines with the affective and cognitive domains of the brain [39]. Extended use of PPIs, which suppress gastric acid, compromises the integrity of the intestinal barrier, leading to a modification in the composition of the microbiota in the small intestine and ultimately causing intestinal dysbiosis [51]. Prolonged administration of PPIs alters the composition of the gastrointestinal microbiome, distinguished by an increased abundance of bacterial taxa, including Gammaproteobacteria, Enterobacteriacea, and Clostridium difficile [59]. The efficacy of PPI medications is enhanced through the usage of probiotic nutrition. In addition, probiotic supplements have shown promise in alleviating intestinal dysbiosis and the negative consequences of extended use of PPI [52]. The activation of microglia, which are innate immune cells of the brain, by Aβ plaque deposits induces an inflammatory response and initiates cognitive impairments in the central nervous system [68]. Abbreviations: PPI: Proton pump inhibitor; Aβ: Amyloid-β plaque.
