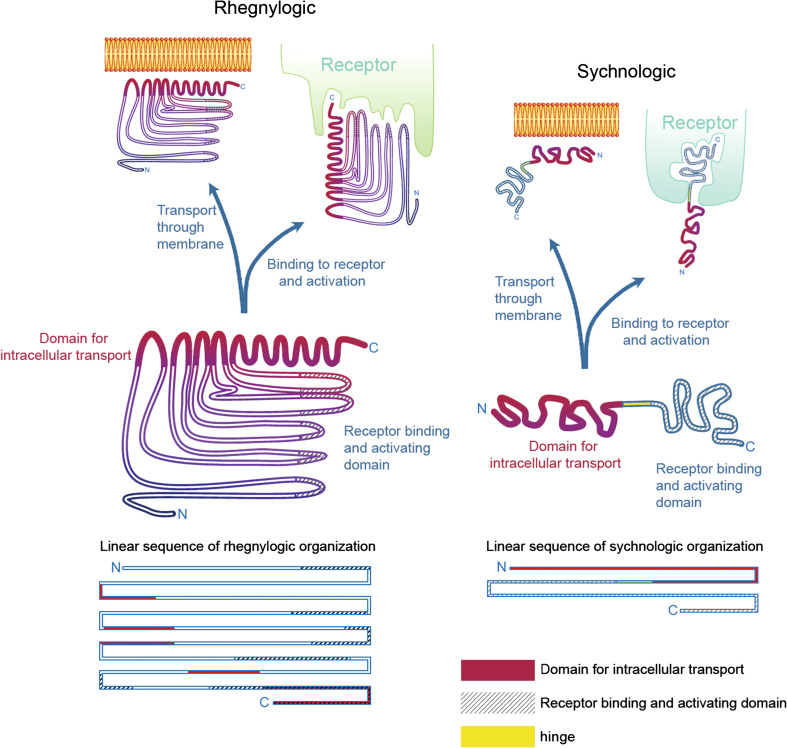
Fig. 1.
Discontinuous (rhegnylogic) or continuous (sychnologic) forms of organization of cell-penetrating toxins. Based on Portoghese et al. [27] and Howl et al. [23], proteins and peptides can be organized according to the domains (1) responsible for transport through membranes and (2) capable of binding and activating receptors, in two general ways: either with a discontinuous organization of both these domain sequences, here considered ‘rhegnylogic,’ or by a continuous, or ‘in tandem’ organization of these domains, here called ‘sychnologic.’ In the first case, cell penetration and activation of receptors are inseparable and are often even overlapped as illustrated by a coiled region at the C-terminus of the left protein structure. Moreover, sequences must be very tightly organized and loss of structural organization can jeopardize protein activity both regarding cell penetration and activation of receptors. Toxins frequently guarantee structure maintenance via disulfide bridges. Indeed, toxin reduction and cleavage of disulfide bridges often causes loss of biological activity. The discovery of proteins with such organization usually requires complex computational analyses of overall properties of amino acid residues and their relationship with other residues of the sequence (QSAR). On the other hand, as shown on the right side of the figure, proteins or peptides can have the domains responsible for cell penetration and receptor activation in separate and independent portions, which can be dissociated and combined with other proteins, maintaining their activity. We have indicated an intermediate joining region that can be thought of as a ‘hinge’ portion that can be particularly flexible, without compromising the independent activities of the transport and receptor binding domains. The binding of transport domains with nanoparticles or receptor binding domains is frequently explored in biotechnology and pharmacology to allow delivery into cells. In this review, we show how peptide sequences derived from toxins, such as NrTPs, SyLop-1 or MCaUF1-9, are used as vectors both by their combination with otherwise non-penetrating sequences or by cross-linking with larger particles