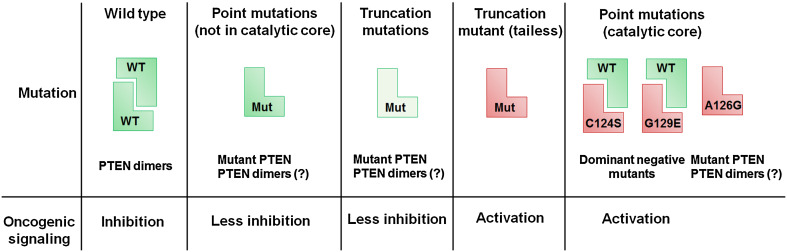
Fig. 4.
Mutations in the PTEN protein generate novel proteoforms with distinct functions. Different PTEN mutations have a varying impact on its function and the resulting phenotype. Point mutations in PTEN, which are outside of the catalytic core, usually have slightly reduced phosphatase activity and/or stability resulting in a mild activation of downstream oncogenic pathways compared to wild-type PTEN. In contrast, mutations in the PTEN catalytic core region result in the production of proteins that are oncogenic (i.e., PTEN C124S or PTEN G129E) or result in the production of PTEN protein with altered enzyme activity (PTEN A126G). Consequently, these point mutations have a worse phenotype compared to PTEN loss perpetuating the concept that PTEN mutations and loss are not synonymous. Truncating mutations in PTEN usually cause a decrease in stability of the PTEN protein, resulting in lower total PTEN levels (indicated in a lighter shade of green). An exception to this is the PTEN C-tail truncated mutant which behaves like an oncogene. Thus, each type of mutation in PTEN gives rise to a functionally and perhaps structurally distinct proteoform