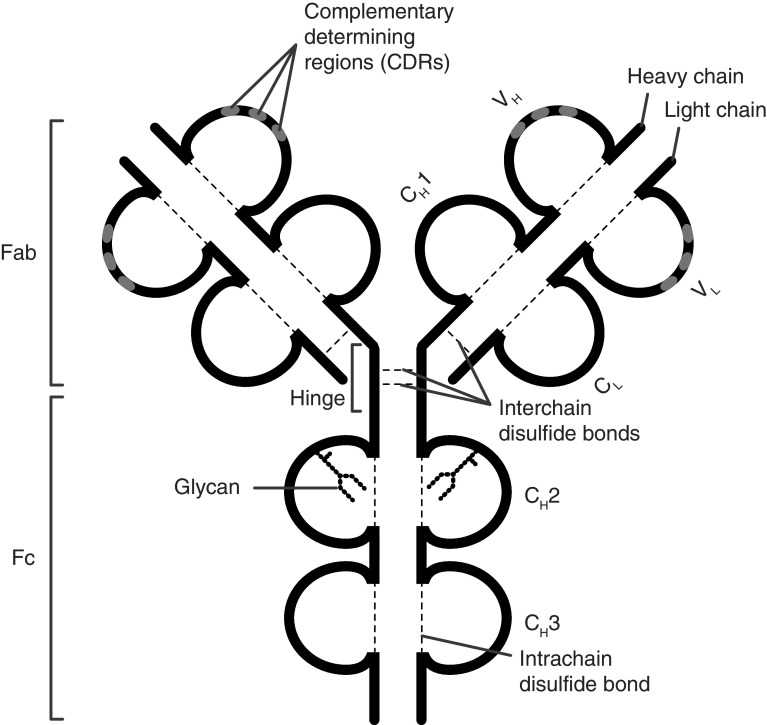
Fig. 1.
Structure of the immunoglobulin G (IgG) molecule. IgG is composed of two heavy and two light chains linked by disulfide bonds. The antigen-binding fragment (Fab) consists of two moieties with identical structure which define the antigen-specificity through their complementarity-determining regions (CDR). The crystallizable fragment (Fc) mediates antibody effector functions through binding to Fc receptors and interaction with the C1q component of the complement system. Each IgG molecule contains a single, highly conserved IgG-Fc N-glycan in each of the two CH2 domains (Fc glycan) and may carry additional glycans in the antigen-binding sites (Fab glycans). CH constant heavy, CL constant light, Fab antigen-binding fragment, Fc crystallizable fragment