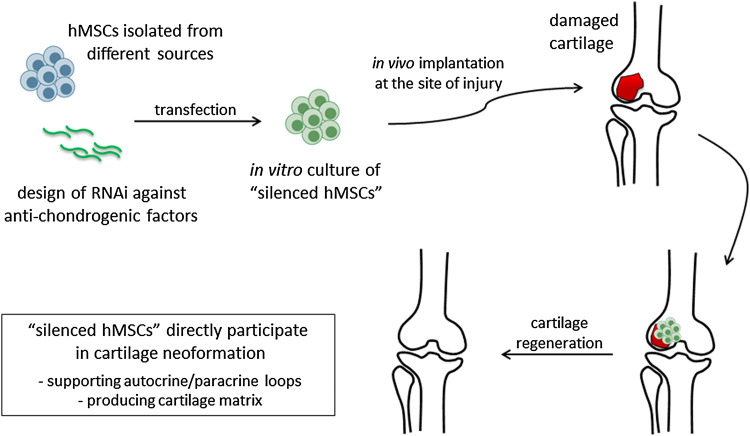
Fig. 1.
Schematic representation of the production and transplantation of “silenced hMSCs” for improved cartilage repair. hMSCs can be collected from different sources including adult niches (e.g., bone marrow and adipose tissue) or perinatal tissues (e.g., placenta, amnios, and Wharton’s jelly of umbilical cord). Following transfection with RNAi molecules, “silenced hMSCs” with enhanced chondrogenic and therapeutic potential are generated. The cells are cultured in vitro for a certain amount of time to obtain an implantable construct, possibly by combination with a scaffold. Different parameters (cell number, oxygen concentration, use of a bioreactor) may be modified to optimize culture conditions and to mimic as much as possible the physiological microenvironment. Following implantation into the site of injury, neoformation of cartilage can be achieved, and tissue functionality can be restored as a result of diverse events potentially supported by the “silenced hMSCs”