Fig. 7.
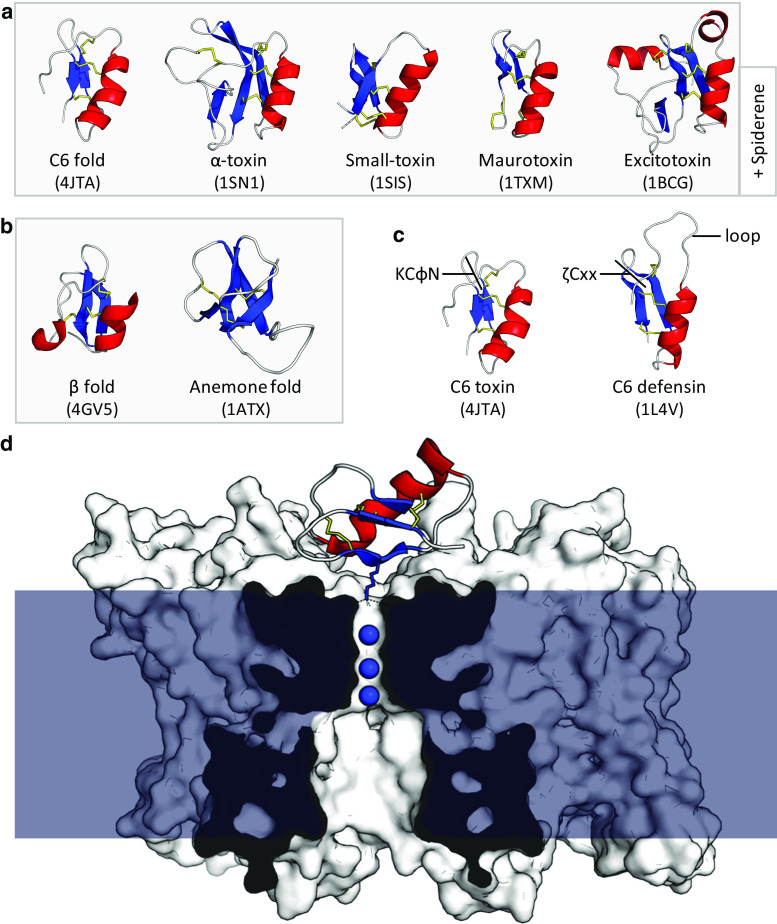
Blocking of ion channels by defensin-like peptides. a The common cis-defensin C6 fold is adapted in some scorpion toxins, along with four toxin-specific structural classes with distinct additional disulphides. A structurally uncharacterised cis-defensin is also present in lynx spider venom (spiderine). b The trans-defensin fold has been recruited to toxic function such as crotamine in snakes, OvDLP from platypus and helofensin from bearded lizards. The anemone fold is also used in sea anemone neurotoxins. c Comparison of the C6 defensin fold with different functions. Toxins contain a conserved KCφN motif, whereas antimicrobial defensins contain the broader ζCxx motif at the same location, in addition to a large, flexible loop (φ = hydrophobe, ζ = hydrophile). d Charybdotoxin binds to the tetrameric K v channel (white surface) and inserts a lysine residue into the first of the channel’s four K+ binding sites, blocking the transport of K+ ions (blue spheres) through the cell membrane (blue) (PDB:4JTA) [93]