Fig. 1.
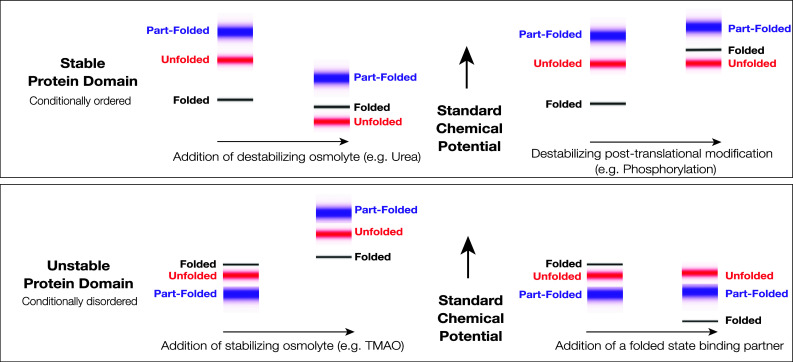
Standard Gibbs energies and protein conformational states. The figure shows schematically the standard chemical potentials (partial molar Gibbs energies) of proteins in the accessible region of the Gibbs energy landscape and their association with protein conformational states. Top A stable protein that folds in two-state fashion under physiological conditions. The low energy states (black) are associated with the very small group of folded state conformations, the higher energy states (red) are associated with the very large group of unfolded state conformations, and the still higher energy states (blue) are associated with the group of partially folded conformations, which may serve as folding transition states. At equilibrium, the folded state conformations are overwhelmingly populated, due to the large difference in the standard chemical potentials of the folded and unfolded states. The folded state is, therefore, the native state. Destabilizing environmental perturbation (e.g., addition of a denaturant like urea) or chemical modification (e.g., phosphorylation) can change the Gibbs energy landscape, allowing the population of the unfolded states. Bottom An unstable, conditionally disordered protein, that has the capacity to fold, but does not do so under physiological conditions. The folded state conformations (black), the unfolded state conformations (red), and the partially folded state conformations (blue) are not well discriminated in the Gibbs energy landscape. Under physiological conditions, the folded state may not be appreciably populated. As depicted here, the partially folded state is the predominant native state. Stabilizing environmental perturbation (e.g., addition of an osmolyte like TMAO or a chemically specific binding partner) can change the Gibbs energy landscape, facilitating partial or complete population of the folded state. In the left panels, the shifts depicted in the figure are consistent in sign with the transfer Gibbs energy measurements carried out by Bolen and coworkers (see [22, 26, 129, 131])
