Fig. 1.
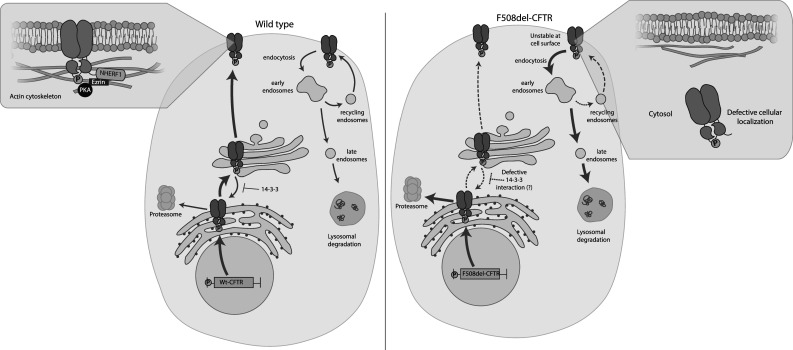
The deletion of phenylalanine at position 508 (F508del) alters phosphoregulation of cystic fibrosis transmembrane conductance regulator (CFTR) trafficking. Left panel: protein kinase A (PKA) phosphorylation promotes CFTR gene expression via the cyclic adenosine monophosphate (cAMP) response element. Phosphorylation also enhances interactions of wild-type CFTR (Wt-CFTR) protein with 14-3-3, which facilitates CFTR exit from the endoplasmic reticulum (ER) and promotes its forward trafficking to the cell surface. Zoom in of the cell surface: phosphorylation enhances the interaction between CFTR and the Na+/H+ exchanger regulatory factor (NHERF1)–ezrin–actin complex which stabilizes the protein at the cell surface. Ezrin also brings PKA closer to CFTR which is essential for regulating channel gating. At the cell surface, CFTR can be internalized by endocytosis into early endosomes which can then recycle back to the cell surface or undergo lysosomal degradation. PKA phosphorylation can also enhance cell surface expression of CFTR by promoting recycling. Right panel: the major population of F508del-CFTR is retained in the ER and undergoes ER-associated degradation via the proteasome. A limited number of F508del-CFTR protein can escape the ER and reach the Golgi apparatus; however, aberrant exposure of “retention motifs” redirects the protein back to the ER. F508del-CFTR may also have impaired interactions with 14-3-3 due to defective phosphorylation of the mutant, resulting in decreased forward trafficking. Zoom in of the cell surface: the small population of F508del-CFTR that reaches the cell surface is unstable and is targeted for degradation via the peripheral protein quality control, possibly due to defective interactions with the NHERF1–ezrin–actin complex. The phosphorylation defect also results in reduced recycling leading to decreased cell surface expression of F508del-CFTR. Not depicted for clarity: CFTR trafficks within vesicles in the cell
