Abstract
The Type-I bone morphogenetic protein receptors (BMPRs), BMPR1A and BMPR1B, present the highest sequence homology among BMPRs, suggestive of functional similitude. However, sequence elements within their extracellular domain, such as signal sequence or N-glycosylation motifs, may result in differential regulation of biosynthetic processing and trafficking and in alterations to receptor function. We show that (i) BMPR1A and the ubiquitous isoform of BMPR1B differed in mode of translocation into the endoplasmic reticulum; and (ii) BMPR1A was N-glycosylated while BMPR1B was not, resulting in greater efficiency of processing and plasma membrane expression of BMPR1A. We further demonstrated the importance of BMPR1A expression and glycosylation in ES-2 ovarian cancer cells, where (i) CRISPR/Cas9-mediated knockout of BMPR1A abrogated BMP2-induced Smad1/5/8 phosphorylation and reduced proliferation of ES-2 cells and (ii) inhibition of N-glycosylation by site-directed mutagenesis, or by tunicamycin or 2-deoxy-d-glucose treatments, reduced biosynthetic processing and plasma membrane expression of BMPR1A and BMP2-induced Smad1/5/8 phosphorylation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-017-2488-y) contains supplementary material, which is available to authorized users.
Keywords: Bone morphogenetic protein, ER translocation, N-Glycosylation, Disulfide bond formation, Intracellular localization, Ovarian cancer
Introduction
The transforming growth factor β (TGFβ) superfamily (TGFβ_SF) of ligands and receptors plays critical roles in health and disease [1–4]. Transduction of signals elicited by TGFβ_SF ligands initiates with formation of a hexameric complex comprising two Type-II receptors, two Type-I receptors, and a dimeric ligand, at the plasma membrane [5, 6]. Within this complex, Type-II receptors phosphorylate and activate Type-I receptors. Subsequent transduction steps involve the phosphorylation of R-Smads by activated Type-I receptors, assembly and nuclear translocation of complexes formed by R-Smads and Co-Smad, and transcriptional regulation of multiple target genes [7–10]. Specifically, bone morphogenetic proteins (BMPs) and the anti-Müllerian Hormone (AMH) signal via the same Type-I receptors (BMPR1A, BMPR1B, and ACVR1), which activate Smad1/5/8 [11–13]. In addition to the Smad pathway, termed canonical, TGFβ_SF ligands in general, and BMPs in particular, activate non-canonical signaling pathways, involving activation of mitogen-activated protein kinases (MAPKs) or stress-activated protein kinases (SAPKs) [14].
Regulation of the levels of expression of receptors at the plasma membrane is of critical importance in determining responsiveness of cells to TGFβ_SF ligands. The plasma membrane content of TGFβ_SF receptors reflects the integration of biosynthetic processing and trafficking, endocytosis, recycling, and degradation [15]. The mode, extent, and function of the internalization of TGFβ_SF receptors has received considerable attention in recent years (recently reviewed in [16]). Similarly, receptor degradation and the role of specific ubiquitin ligases in this process have been extensively addressed [17]. In contrast, much less is known about the regulation of the biosynthesis, processing, and secretory trafficking of TGFβ_SF receptors, even though differences in the processing among these receptors were identified early on [18, 19].
All Type-I and Type-II receptors of the TGFβ_SF are single-spanning transmembrane receptors with N-terminal domains assuming a luminal orientation in intracellular compartments, and extracellular orientation at the plasma membrane. The translocation of single-spanning transmembrane receptors occurs at the endoplasmic reticulum (ER) and is mediated either by a N-terminal signal peptide in the case of Type-1 transmembrane receptors, or by the transmembrane domain in the cases of Type-2 and Type-3 transmembrane receptors. The distinction between the latter two types depends on the orientation of receptor topology [20]. Until recently, all Type-I and Type-II receptors of the TGFβ_SF were classified as Type-1 transmembrane proteins. Recently, the predicted N-terminal signal peptide sequence of the Type-II AMH receptor (AMHR2) was shown to lack functionality, suggesting that its translocation is mediated by the transmembrane domain [21], thus classifying it as a Type-3 transmembrane receptor.
The majority of secretory proteins and transmembrane receptors are N-glycosylated. This post-translational modification was proposed to regulate the folding, turnover, sorting, and endocytosis of different signaling receptors [22–25]. Within the TGFβ_SF, N-glycosylation influences the membrane expression of TGFβ receptors and the sensitivity of cells to TGFβ stimuli [19, 26]. At the plasma membrane, N-glycosylation of TGFβ receptors regulates their endocytosis and inclusion into lipid- and glycan-mediated microdomains [27, 28]. The modulatory effects of glycosylation on ligand-receptor interactions may depend on the identity of either ligands or receptors. For example, ligand-binding by bone morphogenetic protein receptor II (BMPRII), but not by the closely related activin receptor 2B (ACVR2B), requires N-glycosyation [29]. Conversely, the binding of BMP6 to the Type-I Activin A receptor (ACVR1), but not to the Type-I BMP receptors, BMPR1B or BMPR1A, depends on N-glycosylation of the ligand [30].
Here we compare the biosynthetic processing of BMPR1A and BMPR1B, which present the highest degree of sequence similarity among TGFβ_SF receptors. The functional resemblance of BMPR1A and BMPR1B is apparent in the partially redundant roles they perform in chondrogenesis [31]. However, while BMPR1B-null mice are viable [32], BMPR1A-null mice are embryonic lethal [33]. Moreover, BMPR1A and BMPR1B exhibit differences in affinity to distinct BMP ligands [34], further supporting the notion of different biological roles. In the context of different malignancies (e.g., gliomas), BMPR1A and BMPR1B were proposed to perform different functions (pro-tumorigenic and tumor-suppressor, respectively) [35–38]. In this study, we explore molecular mechanisms of differential regulation of BMPR1A and BMPR1B biosynthesis and biosynthetic processing. We show that BMPR1A and the ubiquitous alternatively spliced isoform of BMPR1B differ in their mode of translocation into the ER and that BMPR1A is N-glycosylated while BMPR1B is not. Moreover, we identify N-glycosylation as a determinant of efficient processing and plasma membrane expression of BMPR1A, influencing its ability to activate Smad1/5/8. Specifically, in ES-2 cells, a BMP-sensitive ovarian cancer cell model, we observed regulatory roles for BMP signaling and BMPR1A expression in cell proliferation and organization of the actin-cytoskeleton; and the positive regulatory role of BMPR1A glycosylation on its ability to mediate Smad1/5/8 phosphorylation.
Results
BMPR1A and BMPR1B differ in the mode of their translocation into the endoplasmic reticulum
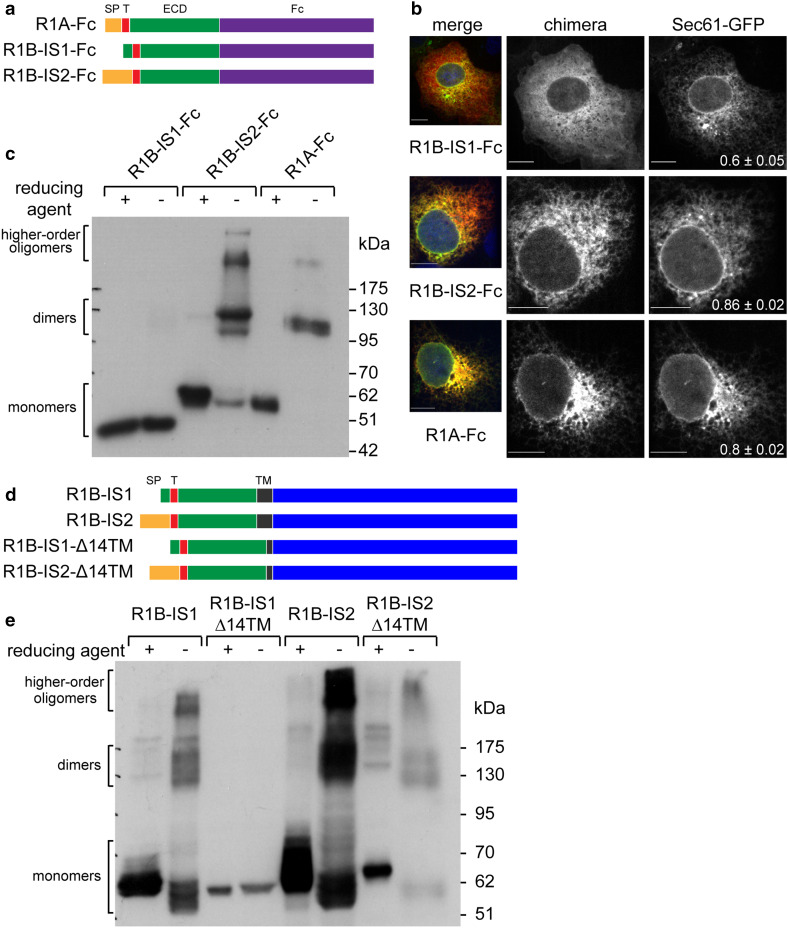
The Type-1A BMP receptor (BMPR1A or R1A) and the Type-1B BMP receptor (BMPR1B or R1B) diverged late in evolution [39]. In the Uniprot database (http://www.uniprot.org), human BMPR1A (P36894) is classified as a single isoform of 532 amino-acids (aa), while there are two alternatively spliced isoforms of human BMPR1B (O00238-1, of 502 aa; O00238-2 of 532 aa). Owing to ubiquitous expression, the shorter isoform is considered the consensus sequence and principal isoform (APPRIS classification of P1, [40]) and denominated here BMPR1B isoform 1 (R1B-IS1), while we denominate the longer isoform BMPR1B isoform 2 (R1B-IS2). Alignment of the protein sequences of R1A with R1B-IS1 or R1B-IS2 showed overall identities of 72% and 70%, respectively (supplementary Fig. S1a). The highest identity between R1A and the R1B receptors is localized to the intracellular domain (ICD, 83% identity), while the extracellular (ECD) and transmembrane (TM) domains showed lower sequence identity (51% and 43%, respectively). The high sequence identity of the ICD, and the lower sequence identity of the ECD, suggests that R1A and R1B may differ in modes of biosynthetic processing or protein–protein interactions mediated by the ECD, while carrying out similar signaling functions following activation (e.g., Smad1/5/8 phosphorylation). The 30 residues which differentiate R1B-IS1 and R1B-IS2 localize to the N-terminal portion of the protein. Analysis of the N-terminal sequences of R1A, R1B-IS2, and R1B-IS1 with SignalP 4.1 Server [41] revealed predicted signal peptides in the two former sequences, while failing to reveal a signal peptide in R1B-IS1 (Supplementary Fig. S1b–d). Since R1B-IS1 is devoid of a predicted signal peptide, we hypothesized that the translocation of its ECD into the lumen of the endoplasmic reticulum (ER) is mediated by its TM domain, defining R1B-IS1 as a Type-3 transmembrane protein. To directly test for the translocation potential of the N-terminal sequences of R1A, R1B-IS1 and R1B-IS2, we generated chimeric constructs consisting of the myc-epitope-tagged ECDs of the receptors fused to the Fcportion of human IgG1 (schematically depicted in Fig. 1a). The chimeric constructs were transiently co-transfected with Sec61-GFP (a translocon component and ER marker), and co-localization of the chimeras and Sec61-GFP was quantified by confocal microscopy. R1A-Fc and R1B-IS2-Fc co-localized with Sec61-GFP in a reticulate distribution pattern [Fig. 1b; Pearson’s correlation coefficient (PCC) ≥ 0.8)], indicating ER localization of the chimeras and in accord with their translocation into the ER. In contrast, immunostaining of R1B-IS1-Fc revealed smooth and continuous intracellular staining, identical to the distribution pattern observed with soluble cytosolic proteins (not shown), and showed a lower degree of co-localization with Sec61-GFP (Fig. 1b; PCC = 0.6). These data suggest that R1A-Fc and RIB-IS2-Fc localize to the ER, while R1B-IS1-Fc retains a cytoplasmic distribution. To test for the access of the ECDs of the chimeric constructs to the oxidizing conditions of the ER lumen, we separated lysates of transfected cells by SDS-PAGE in reducing and non-reducing conditions, and probed for formation of disulfide bonds. Under non-reducing conditions, intramolecular disulfide bonds are detected as a reduction in the apparent molecular weight of monomers (due to the compact structure of the folded ECD), while intermolecular disulfide bonds result in formation of higher-molecular weight oligomers [42]. Both intramolecular and intermolecular disulfide bonds were clearly observable with R1B-IS2-Fc and R1A-Fc, indicating that these proteins translocated into the ER lumen. In contrast, migration of R1B-IS1-Fc in the SDS-PAGE gel was unchanged in reducing and non-reducing conditions, suggesting a lack of disulfide bond formation and no exposure to the oxidizing environment of the ER (Fig. 1c). Together, we interpret these experiments as indicating that R1B-IS1 lacks an N-terminal signal peptide. Since the chimeras are devoid of a TM domain, this construct is not translocated into the ER.
Fig. 1.
The ubiquitous isoform of BMPR1B (R1B-IS1) is a Type-3 transmembrane protein. a Schematic representation of myc-tagged chimeras of the extracellular domain of RIA or R1B isoforms fused to the Fc portion of human IgG1. Signal peptide (SP), orange; myc-tag (T), magenta; extracellular domain (ECD), green; Fc portion of human IgG (Fc), purple. b COS7 cells were co-transfected with Sec61-GFP and with myc-tagged chimeras. At 24-h post-transfection, cells were labeled with anti-myc and alexa-555-conjugated goat-anti-mouse-antibodies. Pearson’s correlation coefficient (PCC) of co-localization of myc-tagged chimeras and Sec61 is presented as mean ± s.e.m. at the bottom right corner of Sec61-GFP image (n > 12 cells; p < 0.01 for comparisons of R1B-ISI-Fc with either R1B-IS-2-Fc or R1A-Fc). Bar 10 µm. c HEK293T cells were transfected (24 h) with myc-tagged chimeras as in a. Cell lysales were separated by SDS-PAGE under reducing (+) or non-reducing (−) conditions and immunoblots were probed with anti-myc antibodies. d Schematic representation of myc-tagged wild-type RIB isoforms and mutant isoforms presenting a 14 aa deletion within the transmembrane domain (R1B-Δ14TM). Signal peptide (SP), orange; myc-tag (T), magenta; extracellular domain (ECD), green; transmembrane domain (TM), black; intracellular domain, blue. e HEK293T cells were transfected with wild-type myc-tagged RIB isoforms or their mutants as in d, and analyzed as in c
ER translocation of single-spanning Type-3 transmembrane proteins, which lack N-terminal signal peptides, is mediated by their TM domain. We hypothesized that if full-length RIB-IS1 is translocated into the ER via its transmembrane domain, shortening of such domain may hamper its translocation-inducing potential. To test this hypothesis, we generated mutant R1B-IS1 and R1B-IS2 constructs in which 14 residues were deleted from the transmembrane domain (denominated Δ14TM; Fig. 1d). Next, we measured the ER translocation of wild-type (WT) R1B-IS1 and RIB-IS2, and of their Δ14TM mutants, by analyzing formation of disulfide bonds. In non-reducing conditions, R1B-IS1-WT formed intramolecular and intermolecular disulfide bonds while R1B-IS1-Δ14TM did not, suggesting that only RIB-IS1 receptors with a full-length TM domain are able to translocate into the ER. In contrast, both RIB-IS2-WT and R1B-IS2-Δ14TM translocated into the ER. These data confirm that R1B-IS2 does not require a full-length TM for translocation (Fig. 1e). Together, these data confirm that ER translocation of R1A and R1B-IS2 is mediated by their signal peptides (Type-1 transmembrane proteins), while R1B-IS1 requires its transmembrane domain (Type-3 transmembrane protein).
Glycosylation of BMPR1A contributes to correct receptor folding and plasma membrane localization
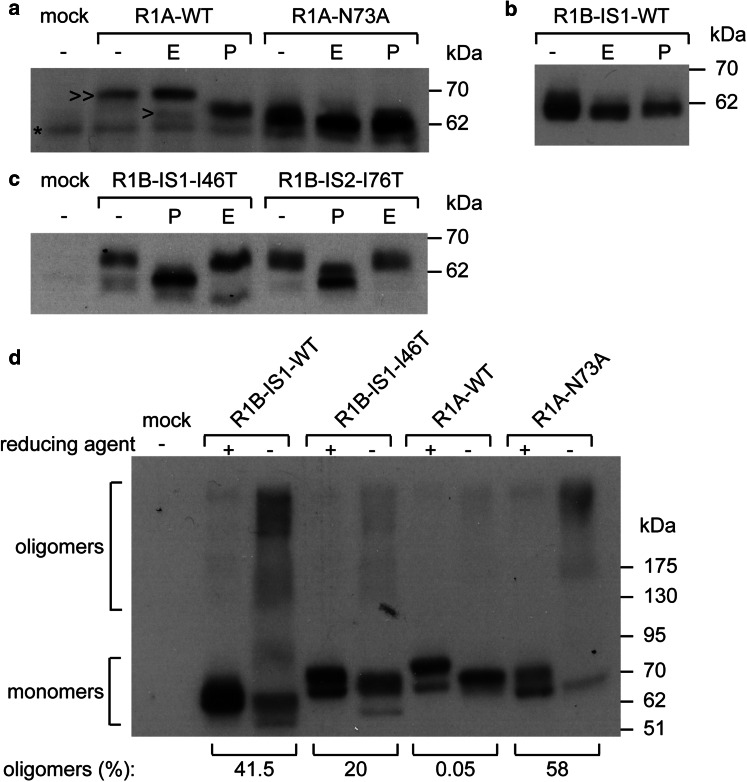
The initial step of N-glycosylation of secreted proteins and transmembrane receptors is commonly co-translational process. The translocation of the luminal N-terminal domain of Type-3 transmembrane proteins is post-translational. As such, Type-3 transmembrane proteins may inherently differ in their tendency towards N-glycosylation. A search in the Uniprot database revealed that ~80% of the human proteins classified as Type-3 transmembrane proteins lack N-glycosylation consensus motifs, suggesting differences in processing of this subset of transmembrane receptors relative to secretory proteins in general, which are commonly N-glycosylated. Comparative analysis of the sequences of the ECDs of R1B and R1A revealed a consensus N-glycosylation site in R1A (73N74N75T) and the absence of such sequence in the analogous position in R1B (44N45N46I; residue numbers are given according to the R1B-IS1 sequence), in accord with their different modes of translocation. To confirm R1A N-glycosylation, we digested myc-tagged R1A (myc-R1A) with Peptide-N-Glycosidase F (PNGaseF, digests all N-linked sugars). This resulted in a collapse in molecular weight of myc-R1A (~3 kDa), confirming its N-glycosylation (Fig. 2a). Treatment with endoglucosydase H (EndoH, digests high-mannose sugar trees) resulted in collapse of a minor portion of the expressed receptor (Fig. 2a). These data suggest that at steady state the majority of RIA in cells is EndoH resistant and has trafficked through the Golgi compartment. To probe if 73N is the glycan acceptor in R1A, we mutated the asparagine to alanine (R1A-N73A). R1A-N73A was insensitive to digestion with either EndoH or PNGaseF and migrated at the same apparent molecular weight as de-glycosylated R1A-WT (Fig. 2a), confirming the N-glycosylation of R1A at the 73N74N75T site. In contrast to R1A, both R1B-IS1 (Fig. 2b) and R1B-IS2 (not shown) were insensitive to digestion with either EndoH or PNGaseF, confirming the lack of N-glycosylation of R1B isoforms.
Fig. 2.
Glycosylation of BMPRIA contributes to its folding. HEK293T cells were transfected with myc-tagged constructs of: a wild-type RIA (R1A-WT) or its glycosylation-negative mutant (R1A-N73A): b R1B-1S1-WT or c glycosylation-gain mutants of R1B isoforms (R1B-1S1-146T R1B-IS2-176T), Cells were lysed at 24 h post-transfection and lysates were digested with EndoH (E) or PNGaseF (P) and separated by SDS-PAGE. Immunoblots were analyzed with anti-myc antibody. >>, EndoH-resistant receptors containing mature glycosyl chains; >, unglycosylated precursor receptors; *, non-specific band. d HEK293T cells were transfected with HA-tagged R1B-IS1-WT, R1B-IS1-146T, R1A-WT or R1A-N73A. Cell lysates (24 h post-transfection) were separated by SDS-PAGE under reducing (+) or non-reducing (−) conditions. Immunoblots were probed with anti-HA antibodies. Numbers below brackets are the percentage of disulfide-bonded oligomers relative to total amount of receptor signal in the non-reducing lane
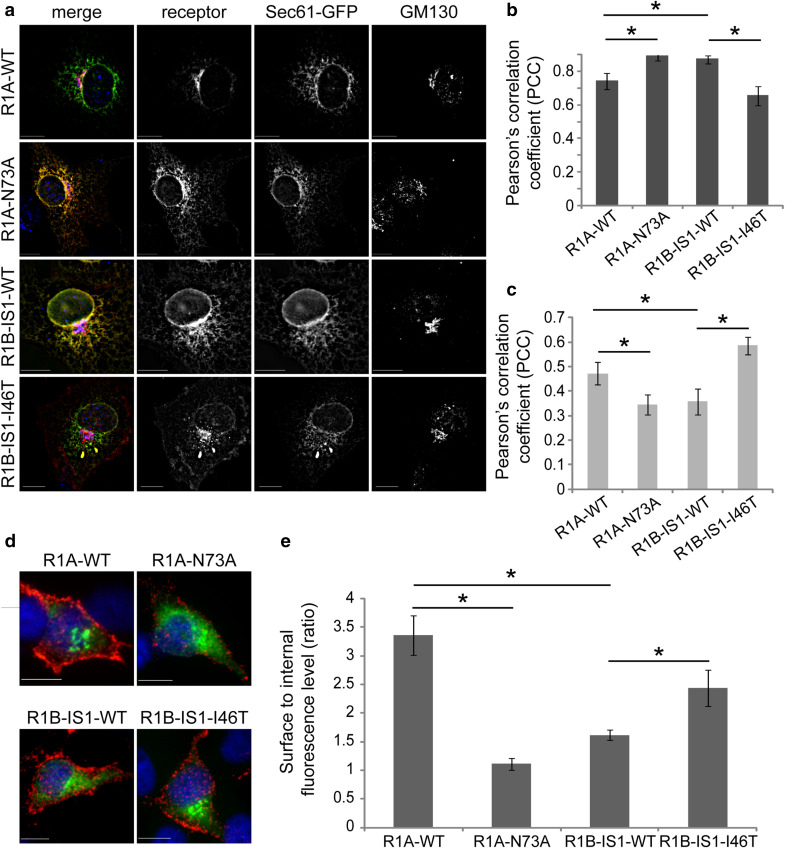
Within the biosynthetic pathway, glycan-lectin interactions influence the folding and processing of glycoproteins. To study if N-glycosylation of R1A, or the lack N-glycosylation of R1B, affects their processing and intracellular distribution we generated R1B mutants in which a functional N-glycosylation site was inserted by substitution of isoleucine to threonine (I46T in R1B-IS1; I76T in R1B-IS2). Results shown in Fig. 2c demonstrate that the mutated site is indeed glycosylated as: (i) R1B-IS1-I46T and R1B-IS2-I76T migrate at a higher apparent molecular weight than their wild-type counterparts, (ii) digestion with PGNaseF leads to a collapse in their apparent molecular weight. Notably, the portion of EndoH-resistant receptors is similar for R1B-IS1-I46T and R1B-IS2-I76T (Fig. 2c). Since acquisition of EndoH resistance occurs at the Golgi compartment, these data suggest that R1B-IS1-I46T and R1B-IS2-I76T are glycosylated and trafficked to the Golgi compartment with similar efficiencies, in spite of their different modes of translocation into the ER. In our previous study [42], we observed that inefficient folding of AMHR2 resulted in formation of higher-molecular weight disulfide-bonded oligomers which were retained in the ER. To test if glycosylation of Type-I BMP receptors modulates their folding, we expressed RIB-IS1-WT, RIB-IS1-I46T, R1A-WT, or R1A-N73A and calculated the portion of receptor which migrates as higher-molecular weight oligomers in non-reducing conditions. Only a minimal portion of R1A-WT formed disulfide-bonded oligomers, suggesting efficient folding of this receptor. In contrast, the majority of the unglycosylated R1A-N73A mutant was present in higher-molecular weight oligomers under non-reducing conditions. A mirror image of these results was observed when comparing RIB-IS1-WT and its glycosylated RIB-IS1-I46T mutant, as the former accumulated as disulfide-bonded oligomers, while the latter was mainly monomeric under non-reducing conditions (Fig. 2d). From such experiments, we conclude that R1A is processed more efficiently than RIB, and that N-glycosylation contributes to its efficient processing. To explore the influence of N-glycosylation on the intracellular distribution of the Type-I BMP receptors, we measured the degree of co-localization of transiently expressed R1A-WT, R1A-N73A, R1B-IS1-WT, and R1B-IS1-I46T with markers of the ER (Sec61-GFP) or Golgi (GM130). N-glycosylated receptors (R1A-WT and R1B-IS1-I46T) presented a prominent peri-nuclear staining pattern and a higher PCC value of co-localization with GM130, while R1B-IS1-WT and R1A-N73A presented a reticulate distribution throughout the entire cell interior and a higher PCC value of co-localization with Sec61-GFP (Fig. 3a–c). These data suggest that N-glycosylation of BMPRs positively correlates with efficient exit from the ER. Retention of receptors in the ER is expected to result in reduced expression at the plasma membrane. To assess if glycosylation of Type-I BMP receptors affects their plasma membrane expression, we performed a quantitative immunofluorescence-microscopy-based surface-to-internal receptor distribution assay. We expressed wild-type myc-tagged R1A or R1B receptors or their mutants, immuno-labeled the cell-surface receptors in live intact cells, and stained internal receptors following fixation and permeabilization of the cells. Removal of the glycosylation site in R1A induced a decrease in the surface-to-internal ratio (R1A-WT > R1A-N73A), while an increase in such ratio was observed upon addition of a glycosylation site to R1B-IS1 (R1B-IS1-I46T > R1B-IS1-WT; Fig. 3d, e). Similarly, an increase (71 ± 12.5%, p < 4 × 10−5) was observed in the surface/internal ratio of expression of R1B-IS2 upon addition of a glycosylation site (R1B-IS2-I76T). Together, these data suggest a contribution of N-glycosylation to the levels of plasma membrane expression of Type-I BMP receptors.
Fig. 3.
Glycosylation of Type-1 BMP receptors regulates their plasma membrane localization. a COS7 cells, co-transfected (24 h) with Sec61-GFP and myc-tagged Type-1 BMP receptors (R1A-WT, R1A-N73A RIB-IS1-WT or RIB-IS1-146T), were immunostained for myc-tag and GM130. Cells were imaged by fluorescence microscopy. Image stacks were de-convolved (NearestNeighbors deconvolution, SlidebookTM) and the PCC of different fluorescence channels was calculated. Micrographs depict a single focal plane of representative cells. Left column are merged images where receptors are in red, Sec6-GFP is in green and GM130 is in blue. Bar 10 µm. b, c Quantification of PCC of fluorescence signals of receptors and Sec61-GFP (b) or receptors and GM130 (c); n > 12 cells for each receptor construct. d Live COS7 cells transfected (24 h) with myc-tagged Type-1 BMP receptors (R1A-WT, RIA-N73A R1B-ISI-WT, or R1B-1S1-146T), were labeled in the cold with chicken-anti-myc and Alexa546-labeled donkey-anti-chicken antibodies, for detection of membrane-external receptors (red). Following fixation, samples were permeabilized and labeled with mouse-anti-myc and Alexa488-labeled goat-anti-mouse antibodies, for detection of internal receptors (green). Micrographs depict single confocal planes of representative cells. Bar 10 µm. e Quantification of the ratio of surface to internal fluorescence signals. Fluorescence signals were calculated from oonfocal z-stacks. Bar graph depicts averaged data (mean ± s.e.m.) of surface to internal ratio; n > 27 cells for each receptor construct
N-Glycosylation regulates BMPR1A processing and signaling in ES-2 ovarian cancer cells
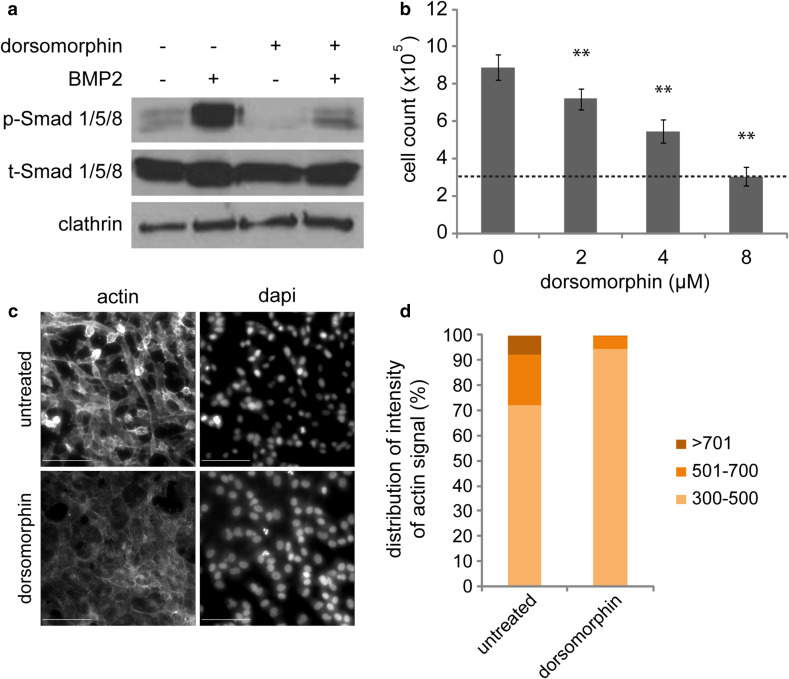
To test for the roles of N-glycosylation of Type-I BMP receptors in a biologically relevant cell model, we initially searched for a BMP-responsive cancer cell line. Signaling by BMP ligands associates with aggressiveness of ovarian cancer cells [43, 44]. Our previous studies identified ES-2 cells as BMP responsive [45], and in vivo xenograft studies demonstrated the aggressiveness of this cellular model in mice [46]. Next, we probed for the activation of BMP signaling and for its role in support of the tumorigenic phenotype in ES-2 cells. ES-2 presented a basal level of phosphorylated Smad1/5/8 (p-Smad1/5/8) when cultured in 10% FBS. Stimulation with BMP2 markedly increased p-Smad1/5/8 levels. Treatment with the Type-I BMP receptor kinase inhibitor dorsomorphin [47] reduced both basal and BMP-induced p-Smad1/5/8 formation (Fig. 4a). Notably, dorsomorphin reduced (in a concentration dependent manner) the proliferation of ES-2 cells (Fig. 4b). To probe for effects of dorsomorphin on motility of ES-2 cells, we imaged untreated or dorsomorphin-treated cell cultures by phase contrast time-lapse microscopy. Untreated ES-2 cells were highly motile (supplementary movie 1), and addition of dorsomorphin resulted in dramatic reduction of cell motility (supplementary movie 2). Having observed the reduction in random cell motility, we assessed for dorsomorphin-mediated effects on cell morphology and on the content of polymerized actin in ES-2 cells by quantitative microscopy measurements of phalloidin staining. Untreated ES-2 cells exhibited mesenchymal-like morphology and foci of concentrated actin, as detected by intensity-based segmentation of phalloidin fluorescence (Fig. 4c, d). In accord with an effect of dorsomorphin on cell motility, dorsomorphin treatment altered the cell–cell contacts and reduced the overall intensity of phalloidin staining and the portion of actin localizing to actin-foci. Together, these data suggest that BMP signaling regulates the proliferation, motility and actin organization of ES-2 cells. However, the effects elicited by dorsomorphin may also be a result of off-target effects of this kinase inhibitor [48]. Thus, to better characterize the effects of BMP signaling and the role performed by type I BMP receptors in this process, we opted to genetically ablate the expression of such receptors on the ES-2 background.
Fig. 4.
Dorsomorphin-mediated inhibition of BMP signaling reduces proliferation and alters actin cytoskeleton organization of ES-2 cells. a ES-2 cells, treated or not with 8 µM dorsomorphin (48 h), were serum-starved (2 h) and stimulated (or not) with 100 ng/ml BMP2 (45 min). Cell lysates were separated by SDS-PAGE and immunoblotted for p-Smad1/5/8, t-Smad1/5/8, and clathrin (loading control). b Dorsomorphin inhibits proliferation of ES-2 cells. 300,000 ES-2 cells were plated in 6-well plates and treated with indicated concentrations of dorsomorphin. Graph depicts average ± s.e.m. of number of cells at 48 h in each condition (n = 3; dashed line depicts initial cell number). c, d Dorsomorphin alters organization of actin cytoskeleton in ES-2 cells. ES-2 cells, treated (or not) with 4 µM dorsomorphin (48 h), were stained with Alexa-546-conjugated phalloidin and DAPI. c Representative micrographs of cell fields. Bar 100 µm. d Quantification of the distribution of intensity of actin signals. Alexa-546 Phalloidin signals were intensity-based segmented into three intensity categories. Graph depicts the percentage of Alexa-546 Phalloidin signal in each category from multiple fields (n = 12). Increase in low intensity category, and concomitant decreases in higher intensity categories were significant for dorsomorphin vs. untreated cells (p < 0.05; n = 12)
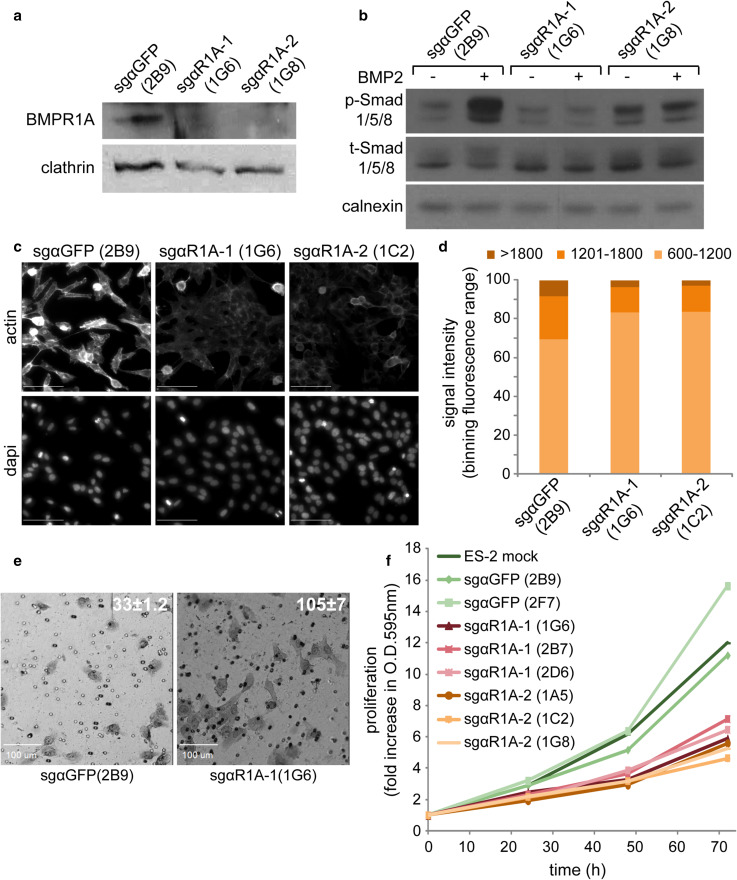
Q-RT-PCR measurements showed that R1A is expressed in ES-2 cells (data not shown). Based on these and the above-shown data, we hypothesized that interference with R1A expression or processing may reduce the BMP-responsiveness of ES-2 cells and diminish the contribution of BMPs to their aggressive cancer phenotype. To probe for the contribution of R1A to the BMP response of ES-2 cells, we opted to knockout R1A with CRISPR/Cas9 [49]. To this end, we employed two different sgRNAs targeting R1A, to minimize potential contribution of off-target effects. We generated retroviral vectors encoding for the sgRNAs and for puromycin resistance, infected ES-2 cells and selected for puromycin-resistant cells. After preliminary experiments showing the knockout potential of the sgRNAs (not shown) and to study homogenous cell populations, we isolated multiple single-cell clones of each of the R1A-knockout cell pools. As control, we infected ES-2 cells with a retrovirus encoding an irrelevant sgRNA sequence (against GFP) and isolated, in parallel, clones from puromycin-resistant cell pools. Immunoblot analysis of endogenous R1A expression in representative clones (sgαGFP(2B9), sgαR1A-1(1G6) and sgαR1A-2(1G8)) demonstrated a sharp reduction in R1A expression following knockout in both sgαR1A clones as compared to the sgαGFP(2B9) clone (Fig. 5a). Importantly, no increase in phosphorylation of Smad1/5/8 following BMP2 stimulation was observed in the sgαR1A-1(1G6) and sgαR1A-2(1G8) clones (Fig. 5b), indicating a prominent role for BMPR1A in the response of ES-2 cells to BMP2. Similarly to the effects induced by dorsomorphin in wild-type ES-2 cells, the sgαR1A-1(1G6) and sgαR1A-2(1G8) clones exhibited reduced portion of actin localizing to actin foci, when compared to the sgαGFP(2B9) clone (Fig. 5c, d). Next, we probed for cellular motility by time-lapse phase contrast microscopy. These experiments revealed a greater motility of sgαGFP(2B9) cells as compared to sgαR1A cells (shown here sgαR1A-1(1G6), supplementary movies 3–4). However, sgαR1A were not completely immobile as dorsomorphin-treated ES-2 cells, stimulating us to better characterize the effects of BMPR1A knockout on the motility and invasiveness of ES-2 cells. Initially, we measured the average velocity of single wild-type ES-2, sgαGFP(2B9) or sgαR1A(1G6) cells in time-lapse sequences. Such analysis revealed a lack of significant differences in the velocity of movement between ES-2 (0.61 ± 0.01 μm/min) and sgαGFP(2B9) cells (0.76 ± 0.03 μm/min), while the velocity of sgαR1A(1G6) cells was significantly lower (0.44 ± 0.01 μm/min; p < 0.02, n > 20 cells). Next, we measured the wound healing ability of the different cell types in a scratch-based assay. Here, no significant differences were observed between the three cell types (data not shown). To characterize the role of BMPR1A expression on the invasiveness of ES2-based cells, we plated, equal numbers of sgαGFP(2B9) or sgαR1A(1G6) cells on Matrigel™ transwells and quantified the number of invading cells at 20-h post-plating. In contrast to the decreased non-directional motility of sgαR1A(1G6), a significantly higher number of sgαR1A(1G6) cells invaded through the Matrigel™ as compared to sgαGFP(2B9) cells (Fig. 5e). Taken together, these data point to regulatory roles performed by BMPR1A on the motility and invasiveness of ES-2 cells, and to the complexity of such regulation, resulting in contrasting results when measuring non-directional two-dimensional motility or invasiveness. Next we assessed for putative regulation of cell proliferation by BMPR1A expression. Methylene-blue assessment of the proliferation rate of multiple CRISPR/Cas9-mediated knockout clones revealed the segregation between sgαGFP clones which proliferated with similar kinetics as wild-type ES-2 cells, and sgαR1A clones which presented significantly reduced proliferation rates (Fig. 5f). Together, these data confirm the contribution of R1A expression to the BMP response of ES-2 cells, and suggest that BMP signaling differentially contributes to tumorigenic features (proliferation, morphology, motility and invasiveness) of this model of ovarian cancer.
Fig. 5.
CRISPR/Cas9-mediated knockout of R1A reduces BMP2-responsiveness and proliferation, and alters actin cytoskeleton organization and invasiveness of ES-2 cells. ES-2 cells were infected with retroviral vectors encoding CRISPR/Cas9 sgRNAs targeting RIA (sgαR1A-1 or sgαR1A-1) or GFP (sgαGFP, non-specific control), as described in “Materials and methods”. Single-cell clones were selected and analyzed. a Immunoblot analysis of endogenous R1A expression in representative clones. Clathrin is shown as loading control. b R1A knockout inhibits the responsiveness of ES-2 cells to BMP2 stimuli. Representative clones of R1A knockout or control cells were serum-starved for 1 h and stimulated with 20 ng/ml BMP2 for 1 h. Following separation of lysates by SDS-PAGE, immunoblots were probed for p-Smad1/5/8, t-Smad1/5/8 and calnexin (loading control). c, d R1A knockout alters organization of actin cytoskeleton. Indicated clones were processed and analyzed for distribution of intensities of phalloem staining as in Fig. 4c–d, with exception of differences in phalloidin staining intensity categories. Increase in percentage in low intensity category, and concomitant decreases in higher intensity categories were significant for both sgαR1A clones vs. sgαGFP clone (p < 0.05, 1-tailed Student’s t test; n > 12 images/clone). e R1A knockout increases the invasiveness of ES-2 cells. Representative images of sgαR1A clone vs. sgαGFP clone analyzed by MatrigelTM invasion assay 20-h post-seeding. Numbers represent the average number of cells that penetrated MatrigelTM membrane in 0.5 mm × 0.5 mm microscopic field (p < 0.01, n = 16 fields). f R1A knockout inhibits proliferation of ES-2 cells. Multiple single-cell clones of R1A-knockout or sgαGFP-control ES-2 cells were assessed for cell proliferation with Methylene Blue assay (see “Materials and methods”). Graph depicts the average optical densities (O.D. = 595 nm; n = 6)
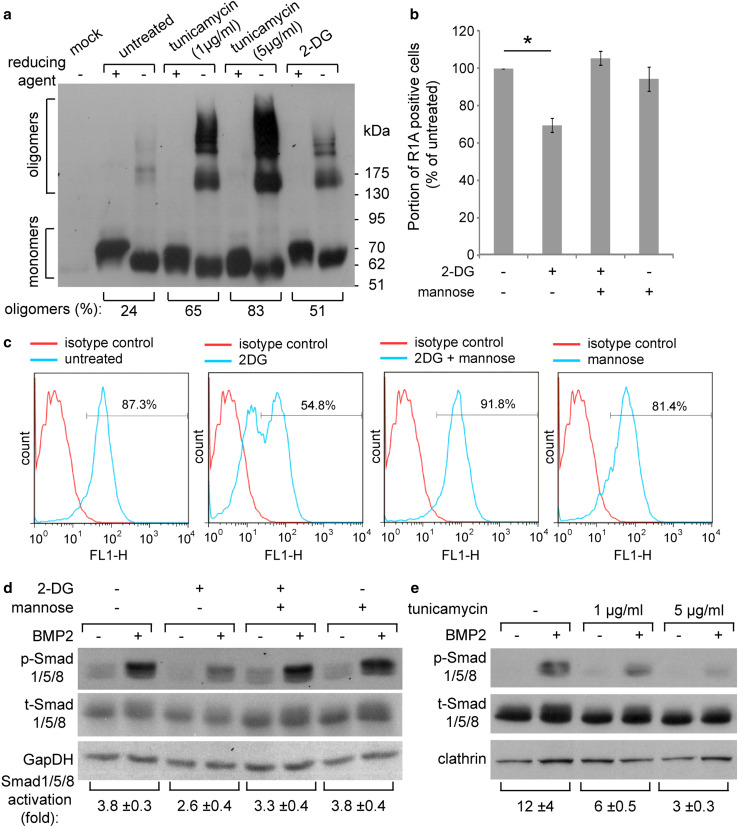
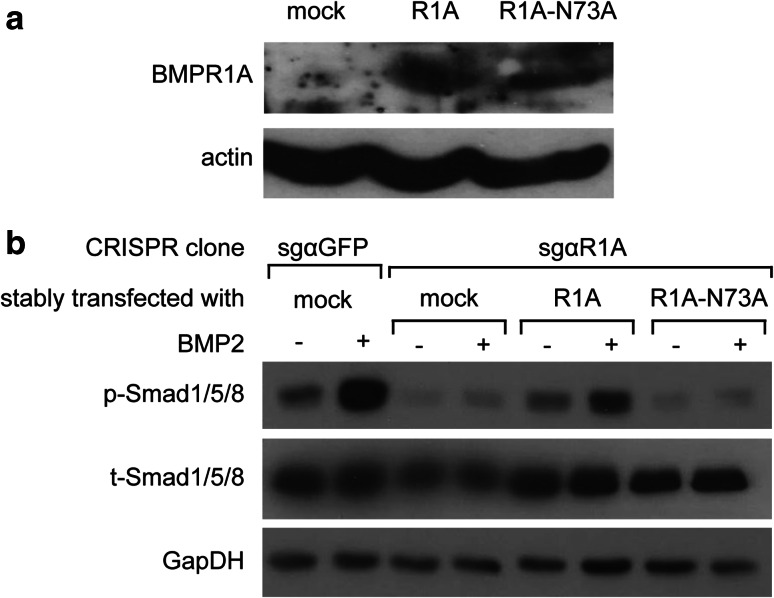
Having identified N-glycosylation as a regulatory determinant of the expression and processing of Type-I BMP receptors, we next addressed the role of N-glycosylation on the processing and plasma membrane expression of BMPR1 receptors in ES-2 cells. Initially, we measured the surface expression of the different receptor constructs (R1A-WT, R1A-N73A, R1B-IS1-WT, and R1B-IS1-I46T) in transiently transfected ES-2 cells by fluorescence activated cell sorting (FACS). Since differences in such levels may result from either differences in transfection efficiency or receptor processing/maturation, we employed the same transfected cultures for assessment of the levels of expression of the receptors, and calculated the ratio of (FACS signal)/(immunoblot signal). These analyses revealed the positive regulation of membrane expression of BMPR1A or BMPR1B by N-glycosylation (Supplementary Fig. S2). To further analyze the role of N-glycosylation on the processing of these receptors in ES-2 cells, we opted for chemical perturbation of N-glycosylation with 2-deoxy-d-glucose (2-DG), as 2-DG is being explored as an anti-cancer compound [50], and addition of excess mannose reverses its N-glycosylation inhibitory effects [51]. To complement studies with 2-DG, we employed tunicamycin, which inhibits the transfer of N-acetylglucosamine 1-phosphate to dolichol monophosphate and fully blocks N-glycosylation. ES-2 cells were transiently transfected with myc-R1A, treated or not with tunicamycin or 2-DG, and analyzed by reducing or non-reducing SDS-PAGE. Both N-glycosylation-perturbing treatments resulted in accumulation of higher-molecular weight disulfide-bonded oligomeric forms of the receptors (Fig. 6a), showing that N-glycosylation is required for the proper folding of R1A in ES-2 cells. In accord with the selective susceptibility of R1A to this regulation (relative to the non-glycosylated R1B), the formation of disulfide-bonded R1B oligomers was not affected by either tunicamycin or 2-DG (Supplementary Fig. S3). To probe if inhibition of N-glycosylation interferes with the plasma membrane expression of endogenous R1A, we measured its membrane expression levels by FACS under different treatment conditions. 2-DG resulted in a decrease in the membrane expression of endogenous R1A (Fig. 6b, c). Since BMPR1A performs a central role in the response of ES-2 cells to BMP2, and BMPR1A processing is susceptible to treatments which inhibit N-glycosylation, we next probed for the effects of 2-DG (±mannose) or tunicamycin on the phosphorylation of Smad1/5/8 in response to BMP2. Stimulation of untreated ES-2 cells with BMP2 (1 h) resulted in robust increase in p-Smad1/5/8 levels, while treatment of the cells with either 2-DG (Fig. 6d) or tunicamycin (Fig. 6e) reduced p-Smad1/5/8 formation. Importantly, co-treatment of 2-DG and mannose rescued membrane expression of endogenous R1A (Fig. 6b, c) and p-Smad1/5/8 formation (Fig. 6d), confirming the contribution of the inhibition of N-glycosylation to the 2DG-induced reduction in p-Smad1/5/8 formation. Since the effects of glycosylation-perturbing drugs may be pleotropic, we next opted to restore the expression of CRISPR/Cas9-resistant glycosylation-competent or glycosylation-deficient R1A mutants to sgαR1A(1G6) cells and measure Smad1/5/8 phosphorylation in response to BMP2 stimuli. In these experiments, a significant increase in p-Smad1/5/8 was only observed in the cells expressing the glycosylation-competent R1A construct, directly confirming the importance of N-glycosylation for R1A function (Fig. 7). Taken together, our data show the central role played by R1A in the BMP responsiveness of ES-2 cells, and the requirement of N-glycosylation of this receptor for its efficient membrane expression and signaling functions.
Fig. 6.
Inhibition of N-glycosylation interferes with R1A processing, reduces its plasma membrane expression, and diminishes BMP2 responsiveness in ES-2 cells. a ES-2 cells were transfected with myc-tagged R1A. At 6-h post-transfection cells were submitted (or not) to treatment with tunicamycin (at indicated concentrations) or 8 mM 2-DG. Cell lysates (24-h post-transfection) were separated by SDS-PAGE under reducing (+) or non-reducing (−) conditions. Immunoblots were probed with anti-myc antibodies. Numbers below brackets are the percentage of disulfide-bonded oligomers relative to total amount of receptor signal in the non-reducing lane. b, c 2-DG reduces membrane expression of endogenous R1A. FACS analysis of surface expression of endogenous R1A. ES-2 cells were treated with 8 mM 2-DG, 1 mM mannose or both for 16 h. Surface-localized R1A was detected by FACS following labeling at 4 °C with anti-R1A and alexa488-conjugated secondary antibodies. Isotype control/alexa488-conjugated secondary antibodies were employed for background determination. b Bar graph depicts relative portion of cells with specific R1A staining (mean ± s.e.m.; *p < 0.05); percentage of positive cells in untreated sample was taken as 100% in each independent experiment. c Representative FACS histograms. d 2-DG inhibits Smad1/5/8 activation. ES-2 cells were treated as in b, serum-starved for 1 h and stimulated with 20 ng/ml BMP2 for 1 h. Immunoblots were probed with anti-p-Smad1/5/8, anti-t-Smad1/5/8 and anti-GapDH (loading control) antibodies. e Tunicamycin inhibits Smad1/5/8 activation. ES-2 cells were treated with the indicated concentrations of tunicamycin for 16 h, serum-starved for 1 h and stimulated with 20 ng/ml BMP2 for 1 h. Immunoblots were probed with anti-p-Smad1/5/8, anti-t-Smad1/5/8 and anti-clathrin (loading control) antibodies
Fig. 7.
Glycosylation of BMPR1A is crucial for the responsiveness of ES-2 cells to BMP2 stimulation. CRISPR/Cas9-mediated BMPR1A knockout ES-2 cells [clone sgαR1A-1(1G6)] were transfected with untagged R1A-WT or R1A-N73A and selected for stable expression as described in “Materials and methods”. a Immunoblot analysis of R1A expression (endogenous or transfected, as indicated). Actin is shown as loading control. b Representative clones of R1A or GFP knockout, or R1A knockout cells stably expressing R1A-WT or R1A-N73A were serum-starved for 1 h and stimulated with 20 ng/ml BMP2 for 1 h. Following separation of lysates by SDS-PAGE, immunoblots were probed for p-Smad1/5/8, t-Smad1/5/8, and GpaDH (loading control)
Discussion
The dependence of signaling by ligands of the TGFβ_SF on cellular context is a broadly accepted concept [1, 16]. Biosynthesis, processing, and secretory trafficking of receptors are all affected by cellular context and offer multiple instances for regulation of the levels and functional status of signaling receptors at the plasma membrane. In extreme cases, disease-related mutations (e.g., occurring in pulmonary arterial hypertension or hereditary hemorrhagic telangiectasia) render mutated receptors of the TGFβ_SF unsuitable for processing and secretory trafficking and result in their intracellular retention. This retention results in the insensitivity of cells to BMP/Activin stimuli [52–54]. The cellular machineries which regulate biosynthesis, processing, and secretory trafficking of transmembrane receptors require discrete molecular motifs in receptor sequences (e.g., signal peptide or glycosylation consensus sequences). Thus, even in highly similar receptors (e.g., BMPR1A and BMPR1B) the presence or absence of such motifs may suffice for differential susceptibility to alterations in cell context. Notably, the median expression of alpha-1,3-mannosyltransferase (ALG3), an enzyme involved in the initial steps of N-glycosylation in the endoplasmic reticulum [55], is highest in ovarian cancer (as compared to other malignancies, analysis carried out with cBioPortal, [56, 57]). Accordingly, a considerable percentage of ovarian cancer patients overexpress ALG3 at either genomic or mRNA levels (Supplementary Fig. S4), suggesting that modified regulation of N-glycosylation may characterize a considerable portion of ovarian cancer patients.
A main finding of the present study is that type I BMP receptors, BMPR1A and BMPR1B, differ in sequence by the presence of signal peptide and consensus N-glycosylation motif only in BMPR1A. The presence of these motifs determines the mode of ER translocation, efficiency of folding, and steady state intracellular distribution of these receptors; and renders them susceptible to differential regulation at the level of biosynthesis, processing and early secretory trafficking. This finding is based on the following lines of evidence: (i) BMPR1B required its transmembrane domain for ER translocation, in contrast to BMPR1A, which was translocated by its extracellular domain; (ii) N-glycosylation of BMPR1A (or of BMPR1B mutants in which a N-glycosylation signal was inserted) prevented formation of disulfide-bonded receptor aggregates and allowed for receptor maturation, secretory trafficking and membrane expression; supporting in this manner the signaling potential of BMPR1A. Importantly, exogenous expression of glycosylation-competent BMPR1A, but not glycosylation-defective BMPR1A, significantly restored the Smad1/5/8 response to BMP2 stimuli in BMPR1A-knockout ES-2 cells.
Our studies support the notion that BMP signaling in general, and BMPR1A-mediated signaling in particular, contribute to the regulation of different tumorigenic features of ES-2 cells. Thus, while the proliferation and random motility of ES-2 cells was positively regulated by BMPR1A expression, invasiveness through a three-dimensional gel was negatively regulated by expression of this receptor. Interestingly, a lack of correlation in the regulation of two-dimensional and three-dimensional cell speeds by the expression of cytoskeleton regulators such as p130Cas, zyxin, or vinculin has been recently reported [58, 59]. Further studies will be required to identify the different downstream targets mediating each of these effects. Our results paint a complex picture regarding the prospect of targeting BMP signaling in ovarian cancer, as inhibitory treatments may have contradictory therapeutic effects with dependence on disease stage.
Membrane integration of transmembrane proteins requires translocation of portions of the poplypeptide chain, which may localize differently relative to the translocation motif: C-terminal in case of cleavable signal peptides or signal anchors, N-terminal in case of reverse signal anchors [20]. The molecular requirements of these two modes of translocation are distinct, allowing for differential and cell context-dependent regulation. For example, while the ribosome provides the motive force for co-translational translocation of C-terminal sequences [60], the motive force of post-translational translocation is provided by different molecular mechanisms, e.g., ratchet-like motion mediated by the chaperone BiP [60]. Thus, reductions in BiP availability (e.g., in ER stress [61]) may result in a specific decrease in the ability of the cell to translocate Type-3 transmembrane proteins. Moreover, post-translational translocation requires maintenance of the unfolded state of the N-terminal portion of the protein, which is translated in the cytosol [62]. This requirement renders post-translational translocation susceptible to regulation by cytosolic chaperones which inhibit premature and aberrant folding. Notably, more than 75% of the human proteins listed as Type-3 transmembrane proteins in the Uniprot database have N-terminal domains of 50 amino acids or less (median value 24 amino acids, Supplementary Fig. S5), suggesting an intrinsic tendency for lack of structure in this portion of the protein. The ECD of R1B-IS1 is 126 amino acids long and folds into an intricate structure [63]. However, stabilization of such structure involves disulfide bond formation, which only occurs upon insertion into the ER. We speculate that cellular conditions which alter the reducing character of the cytosol (e.g., oxidative stress) may differentially affect the translocation of Type-1 and Type-3 proteins in general and of the BMPR1A and BMPR1B receptors in particular.
N-Glycosylation of signaling receptors holds the potential of regulation of multiple aspects of their expression and function. Within the TGFβ_SF, at the plasma membrane, glycosylation regulates the distribution of receptors into lipid- or glycan-mediated microdomains and increases the interaction of BMPR2 (but not ACVR2A) with ligands [27–29]. Moreover, fucosylation, which modifies N-glycans at the Golgi compartment, was shown to be required for TGFβ-induced epithelial to mesenchymal transition in renal tubular cells [64]. Importantly, the co-translational nature of N-glycosylation in the ER is thought to promote folding by directly stabilizing polypeptide structures or by mediating interactions of the nascent protein with lectins, glycosidases, and glycosyltranferases [25]. In particular, N-glycosylated proteins are subject to reiterative cycles of calnexin/calreticulin-assisted folding, which may also facilitate the formation/remodeling of disulfide bonds via activity of isomerases such as Erp57 [65]. In the present study, we observed a direct correlation between the presence of an N-glycosylation site in the ECD of wild-type R1A (or in the R1B-I46T glycosylation-gain mutant) and the formation of correct (intramolecular) as opposed to incorrect (intermolecular) disulfide bonds, suggesting a coupling of the two processes. Moreover, we also observed a direct correlation between the glycosylation of receptors and their membrane expression. Thus, unglycosylated BMPR1 isoforms (R1B-IS1-WT and R1A-N73A) demonstrated higher degree of localization to the ER (and lower Golgi and plasma membrane localization) than their glycosylated counterparts (R1A-WT and R1B-IS1-I46T). Accordingly, perturbation of N-glycosylation with either 2-DG or tunicamycin induced the aggregation of R1A, reduced the membrane expression of endogenous R1A and diminished the activation of Smad1/5/8 in ES-2 ovarian cancer cells stimulated with BMP2.
In summary, we propose that the combination of tumor-induced modifications to the biosynthetic processing machineries of cancer cells, and the differential biosynthetic regulation of BMP receptors (or other TGFβ_SF receptors), hold the potential to induce changes to the repertoire of plasma membrane-localized receptors and contribute to the cell context dependency of signaling by TGFβ_SF ligands [1, 16].
Materials and methods
Cell culture and transfections
HEK293T (CRL-3216), COS7 (CRL-1651) cells (A.T.C.C.), and human ovarian cancer ES-2 (CRL-1978) cells [66] were maintained in DMEM (Dulbecco’s modified Eagle’s medium; Gibco®) supplemented with 10% Fetal Bovine Serum (FBS), 25 µg/ml Penicillin, 40 µg/ml Streptomycin, and 5 mM Glutamine (all from Biological Industries, Beit HaEmek, Israel). Transfections were with calcium phosphate (HEK293T), or JetPRIME® (Polyplus; COS7 and ES-2). ES-2 and HEK293T cells were authenticated by STR analysis carried out by Biological Core Facility, the Genomics center, Technion, Israel.
Expression plasmids
All primers employed in mutagenesis and/or cloning are in supplementary Table 1. Myc- and HA-tagged human R1A and mouse R1B-IS1 constructs (both in pcDNA1; [67]) were a gift from Prof. Yoav Henis (Tel Aviv University, Tel-Aviv, Israel). The extracellular domain (ECD) of mouse R1B was made similar to the human R1B sequence by five point-mutation aa substitutions (S6A, I29V, M64L, D115V, K126R) with the QuikChange Lightning Multi-Site-directed Mutagenesis Kit (#210514; Agilent Technologies). Key experiments were done with both mouse and “humanized” R1B constructs and yielded no significant differences. Generation of R1B-IS2 (insertion of 30 N-terminal aa sequence, NCBI Reference Sequence: NM_001256793.1) was by overlapping PCR. R1B-IS2 sequence was cloned to pcDNA3.1b/myc/His (Invitrogen) between EcoRI and XhoI restriction sites. Chimeric constructs of the myc-tagged ECD of R1A, R1B-IS1 and R1B-IS2 fused to the Fc portion of human IgG1 (hinge-CH2-CH3) were generated by overlapping PCR and cloned to pCDNA3.1b/myc/His between BamHI and NotI restriction sites. Human IgG1 heavy chain expression construct (cloned to pCMV/myc/ER between BssHII and XbaI restriction sites) was a gift from Prof. Itay Benhar (Tel Aviv University, Tel-Aviv, Israel), and was used as a template for generation of Fc chimeric constructs. Deletion of 14aa of the transmembrane domain was by overlapping PCR. Point mutations for generation of glycosylation-negative mutant of R1A and glycosylation-gain mutant of R1B were with the QuikChange Lightning Site-directed Mutagenesis Kit (#210518; Agilent Technologies). Synonymous mutations at the seed region of sgαR1A (oligo 1) were inserted to untagged R1A and R1A-N73A with the QuikChange Lightning Multi-Site-directed Mutagenesis Kit (#210514; Agilent Technologies). Sec61-GFP expression vector was a gift from Prof. Gerardo Z. Lederkremer, (Tel-Aviv University, Tel-Aviv, Israel).
Reagents
2-Deoxy-d-glucose (2-DG), dl-Dithiothreitol (DTT), dorsomorphin, N-ethylmaleimide (NEM), mannose, tunicamycin (Sigma-Aldrich); endoglucosidase H (EndoH), peptide-N-glycosidase F (PNGaseF; New England BioLabs); BMP2 (PeproTech).
Immunochemicals
Antibodies were used at WB: 1:1000, IF: 1:100, unless specified otherwise. Mouse anti-myc (9E10 hybridoma; from Prof. Yoav Henis; Tel Aviv University, Tel Aviv, Israel); chicken-anti-myc (1:10; #AB3252, Chemicon International); mouse monoclonal anti-HA tag (WB; #H3663); anti-GM130 (IF, 1:250; #AB52649, Sigma Aldrich); rabbit polyclonal α-HA.11 (IF, 1:350; #PRB101C, Covance); anti-pSmad1/5/8/9 (#13820) anti-calnexin (#2679), anti-clathrin heavy chain (#2410; Cell Signaling); anti-Smad1/5/8/9 (#SC-6031-R), rabbit polyclonal anti-BMPR1A (#SC-20736, WB: 1:500, FACS: 1:100; Santa Cruz). Secondary Alexa 488-, 555- and 647-conjugated goat-anti-mouse (GαM), and goat-anti-rabbit (GαR) IgGs (1:200; Invitrogen-Molecular Probes). Cy-3 conjugated donkey-anti-chicken, Normal Goat Gamma-Globulin (NGG; FACS: 1:56), HRP-conjugated goat-anti-mouse and goat-anti-rabbit secondary antibodies (both 1:12,500 for WB; Jackson Immuno Research).
Cell lysis and immunoblotting
Transiently transfected cells were analyzed 24-h post-transfection. Equal number of cells were lysed in [150 mM NaCl, 10 mM Hepes pH 7.4, 0.5% Igepal CA-630, 1% Triton X-100, protease and phosphatase inhibitors (Sigma-Aldrich)]. The lysis buffer of “non-reduced” samples was supplemented with 25 mM NEM. Sample buffer (10 mM Tris-HCl pH 8, 10% glycerol, 2% SDS) was supplemented with 20 mM DTT for “reduced samples,” or equivalent volume of water for “non-reduced samples”. 100 mM NEM was added to all samples after boiling. Immunoblots are representatives of at least three independent experiments.
Immunofluorescence
At 24-h post-transfection, cells grown on coverslips were washed twice with cold PBS (4 °C), fixed (4% paraformaldehyde, PFA; 20 min; room temperature), blocked and permeabilized (3% BSA, 0.1% triton, in PBS; 1 h), stained with primary antibodies (1% BSA, 0.1% triton, in PBS; 1 h; staining solution) and secondary antibodies (1:200 dilution in staining solution supplemented with 1 µg/mL DAPI stain, Sigma Aldrich; 30 min). Mounting was with Fluorescence Mounting medium (Golden Bridge).
Surface to internal immunofluorescence assay
COS7 cells were transfected with myc-tagged receptor constructs. At 24-h post-transfection, live intact cells were incubated with serum-free DMEM (37 °C, 30 min). After washing twice with cold Hanks’ balanced salt solution supplemented with 20 mM HEPES and 2% BSA, cells were incubated in the same buffer (4 °C, 30 min) with normal NGG (200 μg/ml) to block non-specific binding. This was followed by successive incubations (4 °C, 30 min each, with three washes between incubations; all performed in the cold to enable exclusive cell-surface labeling by the antibodies and to eliminate internalization) with the following: 1–20 μg/ml chicken-anti-myc primary antibody and 2-Cy3-conjugated donkey-anti-chicken antibody. Cells were then fixed/permeabilized (5 min Methanol and 5 min Methanol:Acetone; −20 °C), blocked with 3% BSA and the intracellular population of myc-tagged receptors and cellular DNA were labeled with mouse-anti-myc and Alexa-488-conjugated GαM supplemented with Dapi.
Imaging, acquisition, processing and quantitation
All microscopy experiments were carried out with fully automated-inverted Zeiss 200 M microscope; piezo controlled Z-stage, under the command of Slidebook™. Phase contrast time-lapse sequences and low magnification fluorescence microscopy employed 10× (NA 0.9, Zeiss) lens. High-magnification fluorescence microscopy and spinning disk confocal microscopy employed a 100× lens (NA 1.45, Zeiss). The latter mode of microscopy was with Yokogawa CSU-22 confocal head, solid state lasers (473, 561 and 660 nm) and Evolve camera (Photometrics, pixel size 0.16 microns). Phase contrast microscopy and wide-field fluorescence microscopy were with EZ camera (Photometrics, pixel size of 0.065 microns). Pearson’s coefficient correlation was calculated for regions of interests (500 × 500 pixels) with the statistical tools of Slidebook™. For calculation of signal intensity specific signals were identified by intensity-based segmentation and corrected for contribution of background signal within selected area. For analysis of the distribution of intensities of actin signals (phalloidin staining), the segmentation employed specified upper and lower range fluorescence intensity values, generating in this manner three categories of signal intensities. For cell tracking, sgαR1A(1G6), sgαGFP(2B9), or wild-type ES-2 cells were transiently transfected with a plasmid encoding for the mCherry [68]. At 8- to 12-h post-transfection cells were sparsly re-seeded onto 35-mm dishes. 12-h after re-seeding, cells were imaged by wide-field fluorescence time-lapse microscopy. Single cells were identified by intensity-based segmentation. Object definition, cell tracking and definition of center of mass of each object (for path calculation) were carried out with Slidebook™ software.
Cell proliferation
Two procedures were employed: 1-cell counting; 2-Methylene blue proliferations assay. In the latter, cells were plated overnight in 96-well plates (5 × 103 cells/well, 6 repetitions/time-point/condition) and incubated as specified in the figure legend. Fresh growth medium was replaced every 24 h. Cells were fixed with 4% formaldehyde, stained with 0.5% methylene blue in 0.1 M sodium borate pH 8.5, and dissolved in 0.1 M HCl. Absorbance was measured at 595 nm.
CRISPR/Cas9-based R1A knockout
The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated nuclease (Cas9) system was employed for the knockout of endogenous BMPR1A in ES-2 cells. Two different small guide RNAs (sgRNAs) targeting BMPR1A and one sgRNA targeting GFP (as control; see supplementary Table 1 for primer sequences) were selected from the Human GeCKO library [49] and cloned into pXPR lenti-CRISPR plasmid (encoding for Puromycin resistance) between BsmBI restriction sites. The resulting pXPR lenti-CRISPR plasmids were validated by sequencing and were each co-transfected with helper Gag-Pol and helper VSV-G (envelope) plasmids (all gifts from Prof. Eran Bacharach, Tel-Aviv University, Tel-Aviv) in to HEK293T cells for formation of virions. Medium containing virions was collected from HEK293T cultures at 48 h post-transfection. Medium was filtered through 0.45 mm filter, supplemented with 8 μg/ml Polyberene (Sigma-Aldrich) and used to infect ES-2 cells. Virus-containing medium was replaced by fresh DMEM 2-h post-infection. Infected cells were selected for lentiviral DNA incorporation with 2 μg/ml Puromycin starting at 48-h post-infection. Individual single-cell clones were separated by seeding 0.5 cell/well in 96-well plates. Puromycin-resistant “cell pools” and clones were maintained under Puromycin selection. CRISPR/Cas9-mediated BMPR1A knockout ES-2 cells stably expressing untagged R1A-WT or R1A-N73A were generated by co-transfection of clone sgαR1A-1 (1G6) with the each of the expression vectors above (incorporating synonymous mutations at the seed region of sgαR1A-oligo 1) and an empty pCDNA3.1b/myc-his vector at a ratio of 10:1 respectively. Cells were selected with 2 μg/ml puromycin and 1.5 mg/ml G-418 starting from 48-h post-transfection.
Flow cytometry
Live ES-2 cells (0.5–1 × 106) were re-suspended in PBS, blocked with NGG, prior to labeling of surface-exposed endogenous R1A with anti-BMPR1A antibody and Alexa488-conjugated GαR antibody (all at 4 °C). R1A levels were measured by fluorescence-activated cell sorting (FACS) using a Becton Dickinson FACSort (Mountain View, CA, USA) and the FlowJo™ software. Baseline staining was obtained by staining with nonrelevant isotype control IgG1 antibody in place of anti-BMPR1A.
Invasion assay
1 × 105 cells were seeded on Corning® BioCoat™ Matrigel™ Invasion Chambers, with 8.0 μm pore PET membrane in 24-well cell culture inserts (354480, Corning, USA). 10% FBS was the chemoattractant. Cells were allowed to invade for 20 h and were fixed, stained and counted in at least five 0.5 mm × 0.5 mm microscopic fields by light microscopy in each experiment (n = 3).
Statistical analysis
Statistical significance throughout this manuscript was calculated with the 2-tailed Student’s t test. *p < 0.05; **p < 0.01; n, number of independent experiments or number of analyzed cells in quantitative confocal imaging.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Prof. Eran Bacharach and Dr. Rachel Zamostiano for their assistance in planning and cloning of CRISPR/Cas9 vectors for knockout of R1A and the control vector directed against GFP.
Author contributions
TH, NIS, and ME planned the study. TH, ML, and OD carried out experiments. TH and ME wrote the manuscript.
Abbreviations
- 2-DG
2-Deoxy-d-glucose
- ACVR1/2
Type-I/II activin receptor
- AMH
Anti-müllerian hormone
- AMHR2
Type-II AMH receptor
- BMP
Bone morphogenetic protein
- BMPR1A or R1A
Type-IA BMP receptor
- BMPR1B or R1B
Type-IB BMP receptor
- CRISPR
Clustered regularly interspaced short palindromic repeats
- CRISPR/Cas9
CRISPR-associated nuclease 9
- ECD
Extracellular domain
- EndoH
Endoglucosydase H
- ER
Endoplasmic reticulum
- ICD
Intracellular (cytosolic) domain
- IS1/2
Isoform½
- MAPK
Mitogen-activated protein kinase
- PCC
Pearson’s correlation coefficient
- p-Smad1/5/8
Phosphorylated Smad1/5/8
- PNGaseF
Peptide-N-glycosidase F
- SAPK
Stress-activated protein kinase
- s.e.m.
Standard error of the mean
- sgRNA
Small guide RNA
- sgαGFP
SgRNA targeting GFP
- sgαR1A
SgRNA targeting BMPR1A
- TGFβ
Transforming growth factor beta
- TGFβ_SF
TGFβ superfamily
- TM
Transmembrane domain
- t-Smad1/5/8
Total Smad1/5/8
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nature Rev Cancer. 2013;13(5):328–341. doi: 10.1038/nrc3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16(3):251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, Shi LL. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrlich M, Gutman O, Knaus P, Henis YI. Oligomeric interactions of TGF-beta and BMP receptors. FEBS Lett. 2012;586(14):1885–1896. doi: 10.1016/j.febslet.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich M, Horbelt D, Marom B, Knaus P, Henis YI. Homomeric and heteromeric complexes among TGF-beta and BMP receptors and their roles in signaling. Cell Signal. 2011;23(9):1424–1432. doi: 10.1016/j.cellsig.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296(5573):1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 9.Zi Z, Chapnick DA, Liu X. Dynamics of TGF-beta/Smad signaling. FEBS Lett. 2012;586(14):1921–1928. doi: 10.1016/j.febslet.2012.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19(2):176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16(3):291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Belville C, Jamin SP, Picard JY, Josso N, di Clemente N. Role of type I receptors for anti-Mullerian hormone in the SMAT-1 Sertoli cell line. Oncogene. 2005;24(31):4984–4992. doi: 10.1038/sj.onc.1208686. [DOI] [PubMed] [Google Scholar]
- 13.Gouedard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, Josso N, Massague J, di Clemente N. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J Biol Chem. 2000;275(36):27973–27978. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- 14.Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277(7):5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 15.Zi Z, Feng Z, Chapnick DA, Dahl M, Deng D, Klipp E, Moustakas A, Liu X. Quantitative analysis of transient and sustained transforming growth factor-beta signaling dynamics. Mol Syst Biol. 2011;7:492. doi: 10.1038/msb.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrlich M. Endocytosis and trafficking of BMP receptors: regulatory mechanisms for fine-tuning the signaling response in different cellular contexts. Cytokine Growth Factor Rev. 2016;27:35–42. doi: 10.1016/j.cytogfr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Izzi L, Attisano L. Regulation of the TGFbeta signalling pathway by ubiquitin-mediated degradation. Oncogene. 2004;23(11):2071–2078. doi: 10.1038/sj.onc.1207412. [DOI] [PubMed] [Google Scholar]
- 18.Koli KM, Arteaga CL. Processing of the transforming growth factor beta type I and II receptors. Biosynthesis and ligand-induced regulation. J Biol Chem. 1997;272(10):6423–6427. doi: 10.1074/jbc.272.10.6423. [DOI] [PubMed] [Google Scholar]
- 19.Wells RG, Yankelev H, Lin HY, Lodish HF. Biosynthesis of the type I and type II TGF-beta receptors. Implications for complex formation. J Biol Chem. 1997;272(17):11444–11451. doi: 10.1074/jbc.272.17.11444. [DOI] [PubMed] [Google Scholar]
- 20.Goder V, Spiess M. Topogenesis of membrane proteins: determinants and dynamics. FEBS Lett. 2001;504(3):87–93. doi: 10.1016/S0014-5793(01)02712-0. [DOI] [PubMed] [Google Scholar]
- 21.Belville C, Marechal JD, Pennetier S, Carmillo P, Masgrau L, Messika-Zeitoun L, Galey J, Machado G, Treton D, Gonzales J, Picard JY, Josso N, Cate RL, di Clemente N. Natural mutations of the anti-Mullerian hormone type II receptor found in persistent Mullerian duct syndrome affect ligand binding, signal transduction and cellular transport. Hum Mol Genet. 2009;18(16):3002–3013. doi: 10.1093/hmg/ddp238. [DOI] [PubMed] [Google Scholar]
- 22.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Lederkremer GZ. Glycoprotein folding, quality control and ER-associated degradation. Curr Opin Struct Biol. 2009;19(5):515–523. doi: 10.1016/j.sbi.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Ren Physiol. 2009;296(3):F459–469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 26.Kim YW, Park J, Lee HJ, Lee SY, Kim SJ. TGF-beta sensitivity is determined by N-linked glycosylation of the Type II TGF-beta receptor. Biochem J. 2012;445(3):403–411. doi: 10.1042/BJ20111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306(5693):120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 28.Luga V, McLean S, Le Roy C, O’Connor-McCourt M, Wrana JL, Di Guglielmo GM. The extracellular domain of the TGFbeta Type II receptor regulates membrane raft partitioning. Biochem J. 2009;421(1):119–131. doi: 10.1042/BJ20081131. [DOI] [PubMed] [Google Scholar]
- 29.Lowery JW, Amich JM, Andonian A, Rosen V. N-linked glycosylation of the bone morphogenetic protein receptor Type 2 (BMPR2) enhances ligand binding. Cell Mol Life Sci. 2014;71(16):3165–3172. doi: 10.1007/s00018-013-1541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saremba S, Nickel J, Seher A, Kotzsch A, Sebald W, Mueller TD. Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J. 2008;275(1):172–183. doi: 10.1111/j.1742-4658.2007.06187.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci USA. 2005;102(14):5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The Type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127(3):621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 33.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9(24):3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 34.Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of Type I and Type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271(35):21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Yin F, Fan W, Wang S, Guo XR, Zhang JN, Tian ZM, Fan M. Over-expression of BMPR-IB reduces the malignancy of glioblastoma cells by upregulation of p21 and p27Kip1. J Exp Clin Cancer Res. 2012;31:52. doi: 10.1186/1756-9966-31-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S, Kim M, Totonchy M, Cusack T, Ene C, Ma H, Su Q, Zenklusen JC, Zhang W, Maric D, Fine HA. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13(1):69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo M, Jiang Z, Zhang X, Lu D, Ha AD, Sun J, Du W, Wu Z, Hu L, Khadarian K, Shen J, Lin Z. miR-656 inhibits glioma tumorigenesis through repression of BMPR1A. Carcinogenesis. 2014;35(8):1698–1706. doi: 10.1093/carcin/bgu030. [DOI] [PubMed] [Google Scholar]
- 38.Hover LD, Owens P, Munden AL, Wang J, Chambless LB, Hopkins CR, Hong CC, Moses HL, Abel TW. Bone morphogenetic protein signaling promotes tumorigenesis in a murine model of high-grade glioma. Neuro-oncol. 2015 doi: 10.1093/neuonc/nov310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huminiecki L, Goldovsky L, Freilich S, Moustakas A, Ouzounis C, Heldin CH. Emergence, development and diversification of the TGF-beta signalling pathway within the animal kingdom. BMC Evol Biol. 2009;9:28. doi: 10.1186/1471-2148-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez JM, Carro A, Valencia A, Tress ML. APPRIS WebServer and WebServices. Nucleic Acids Res. 2015;43(W1):W455–459. doi: 10.1093/nar/gkv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 42.Hirschhorn T, di Clemente N, Amsalem AR, Pepinsky RB, Picard JY, Smorodinsky NI, Cate RL, Ehrlich M. Constitutive negative regulation in the processing of the anti-Mullerian hormone receptor II. J Cell Sci. 2015;128(7):1352–1364. doi: 10.1242/jcs.160143. [DOI] [PubMed] [Google Scholar]
- 43.Peng J, Yoshioka Y, Mandai M, Matsumura N, Baba T, Yamaguchi K, Hamanishi J, Kharma B, Murakami R, Abiko K, Murphy SK, Konishi I. The BMP signaling pathway leads to enhanced proliferation in serous ovarian cancer-a potential therapeutic target. Mol Carcinog. 2015 doi: 10.1002/mc.22283. [DOI] [PubMed] [Google Scholar]
- 44.Hover LD, Young CD, Bhola NE, Wilson AJ, Khabele D, Hong CC, Moses HL, Owens P. Small molecule inhibitor of the bone morphogenetic protein pathway DMH1 reduces ovarian cancer cell growth. Cancer Lett. 2015;368(1):79–87. doi: 10.1016/j.canlet.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirschhorn T, Barizilay L, Smorodinsky NI, Ehrlich M. Differential regulation of Smad3 and of the Type II transforming growth factor-beta receptor in mitosis: implications for signaling. PLoS ONE. 2012;7(8):e43459. doi: 10.1371/journal.pone.0043459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 2004;10(6):1032–1042. doi: 10.1016/j.ymthe.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFss and BMP pathways. Cell Signal. 2011;23(11):1831–1842. doi: 10.1016/j.cellsig.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 49.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-d-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355(2):176–183. doi: 10.1016/j.canlet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Ahadova A, Gebert J, von Knebel Doeberitz M, Kopitz J, Kloor M. Dose-dependent effect of 2-deoxy-d-glucose on glycoprotein mannosylation in cancer cells. IUBMB Life. 2015;67(3):218–226. doi: 10.1002/iub.1364. [DOI] [PubMed] [Google Scholar]
- 52.Frump AL, Lowery JW, Hamid R, Austin ED, de Caestecker M. Abnormal trafficking of endogenously expressed BMPR2 mutant allelic products in patients with heritable pulmonary arterial hypertension. PLoS ONE. 2013;8(11):e80319. doi: 10.1371/journal.pone.0080319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sobolewski A, Rudarakanchana N, Upton PD, Yang J, Crilley TK, Trembath RC, Morrell NW. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: potential for rescue. Hum Mol Genet. 2008;17(20):3180–3190. doi: 10.1093/hmg/ddn214. [DOI] [PubMed] [Google Scholar]
- 54.Hume AN, John A, Akawi NA, Al-Awadhi AM, Al-Suwaidi SS, Al-Gazali L, Ali BR. Retention in the endoplasmic reticulum is the underlying mechanism of some hereditary haemorrhagic telangiectasia type 2 ALK1 missense mutations. Mol Cell Biochem. 2013;373(1–2):247–257. doi: 10.1007/s11010-012-1496-3. [DOI] [PubMed] [Google Scholar]
- 55.Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 2013;1833(11):2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mierke CT, Kollmannsberger P, Zitterbart DP, Diez G, Koch TM, Marg S, Ziegler WH, Goldmann WH, Fabry B. Vinculin facilitates cell invasion into three-dimensional collagen matrices. J Biol Chem. 2010;285(17):13121–13130. doi: 10.1074/jbc.M109.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol. 2010;12(6):598–604. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450(7170):663–669. doi: 10.1038/nature06384. [DOI] [PubMed] [Google Scholar]
- 61.Lai CW, Aronson DE, Snapp EL. BiP availability distinguishes states of homeostasis and stress in the endoplasmic reticulum of living cells. Mol Biol Cell. 2010;21(12):1909–1921. doi: 10.1091/mbc.E09-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kida Y, Mihara K, Sakaguchi M. Translocation of a long amino-terminal domain through ER membrane by following signal-anchor sequence. Embo J. 2005;24(18):3202–3213. doi: 10.1038/sj.emboj.7600788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kotzsch A, Nickel J, Sebald W, Mueller TD. Purification, crystallization and preliminary data analysis of ligand-receptor complexes of growth and differentiation factor 5 (GDF5) and BMP receptor IB (BRIB) Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65(Pt 8):779–783. doi: 10.1107/S1744309109024142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin H, Wang D, Wu T, Dong C, Shen N, Sun Y, Sun Y, Xie H, Wang N, Shan L. Blocking core fucosylation of TGF-beta1 receptors downregulates their functions and attenuates the epithelial-mesenchymal transition of renal tubular cells. Am J Physiol Renal Physiol. 2011;300(4):F1017–1025. doi: 10.1152/ajprenal.00426.2010. [DOI] [PubMed] [Google Scholar]
- 65.Jessop CE, Tavender TJ, Watkins RH, Chambers JE, Bulleid NJ. Substrate specificity of the oxidoreductase ERp57 is determined primarily by its interaction with calnexin and calreticulin. J Biol Chem. 2009;284(4):2194–2202. doi: 10.1074/jbc.M808054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chetrit D, Barzilay L, Horn G, Bielik T, Smorodinsky NI, Ehrlich M. Negative regulation of the endocytic adaptor disabled-2 (Dab2) in mitosis. J Biol Chem. 2011;286(7):5392–5403. doi: 10.1074/jbc.M110.161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilboa L, Nohe A, Geissendorfer T, Sebald W, Henis YI, Knaus P. Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol Biol Cell. 2000;11(3):1023–1035. doi: 10.1091/mbc.11.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Viner R, Chetrit D, Ehrlich M, Segal G. Identification of two Legionella pneumophila effectors that manipulate host phospholipids biosynthesis. PLoS Pathog. 2012;8(11):e1002988. doi: 10.1371/journal.ppat.1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.