Fig. 2.
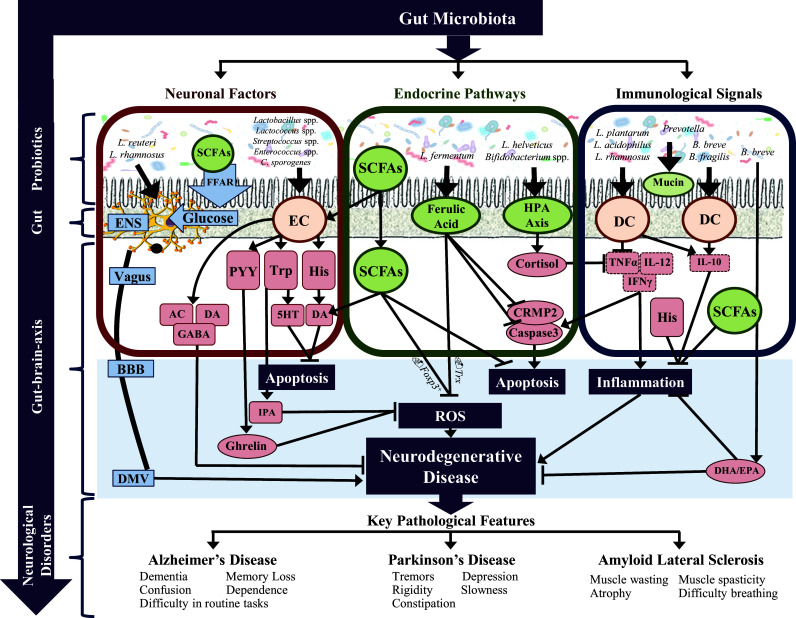
Investigating the mechanisms of probiotic treatment in neurological signaling. The gut microbiota impacts neurological disease via three main modalities: (1) neuronal factors, (2) endocrine pathways and (3) immunological signals. The microbiota present in the gut lumen plays a specific role in influencing these three pathways. (1) Individual bacteria can both produce certain neurotransmitters such as DA and ACh while the same and others stimulate neurotransmitter production via the secretory ECs such as 5HT and GABA. The ECs cells can also produce several neuroactive factors including PYY, Typ and His. The neurotransmitters and neuroactive molecules enter blood circulation and cross the BBB influencing CNS signaling. Some neuroactive components also go further to stimulate the production of gut hormones in the CNS such as ghrelin and IPA that have dual roles in the CNS including neuroprotection. Further, individual microbiota species can directly stimulate electrical signals in the ENS, thereby propagating signals through the vagus nerve to stimulate the DMV. Finally, the microbiota through the production of SCFAs and FFAR signaling, releases glucose which also propagates signals through the ENS. (2) The gut microbiota directly and indirectly produces a battery of endocrine signaling molecules. SCFAs, including propionate, butyrate and acetate, are major signaling molecules produced by the microbiota that have many roles including stimulation of neurotransmitter synthesis in the periphery and centrally, inhibiting ROS production by upregulating FoxP + transcription and inhibiting apoptosis through caspase cascades. A major mechanism instigated by the gut microbiota is HPA axis stimulation and the consequent release of cortisol. Cortisol suppresses the inflammatory response and influences a number of neurological processes. Ferulic acid is another key molecule produced directly by the microbiota that has a variety of functions including suppression of ROS both directly and by indirectly by PRX/Trx signaling, suppression of apoptosis by inhibiting CRMP2 and caspase 3 expression and either directly or indirectly, suppressing the inflammatory response. (3) A healthy gut microbiota suppresses inflammation, both chronic and pathological. The MAMPs such as LTA and SlpA on the surface of microbiota directly stimulate receptors (TLR and ICAM, respectively) on immunological cells such as DCs. This interaction propagates an anti-inflammatory response with an upregulation of anti-inflammatory factors (IL-10 and IL-4) while suppressing proinflammatory cytokines (TNFα, IL-1β and IL-6). In addition, some microbiota also directly suppresses proinflammatory cytokines such as the action of B. animalis on IL-6. Finally, the gut microbiota influences the production of mucin, an inhibitory chemical gel that blocks the penetrance of pathogens through the gut. 5HT serotonin, Ach acetylcholine, BBB blood–brain barrier, CRMP2 collapsin response mediator protein family, DA dopamine, DHA docosahexaenoic acid, DMV dorsal motor nucleus of the vagus, EC enterochromaffin cell, ENS enteric nervous system, EPA eicosapentaenoic acid, FFAR free fatty acid receptor, GABA gamma-aminobutyric acid, His histamine, HPA hypothalamic–pituitary–adrenal axis, IFNγ interferon gamma, IL-10 interleukin 10, IL-12 interleukin 12, IPA indole-3-propionic acid, NA noradrenaline, PRX peroxiredoxin, PYY peptide YY, ROS reactive oxygen species, SCFAs short-chain fatty acids, TNFα tumor necrosis factor alpha, Trp tryptophan, Trx thioredoxin
