Mitochondrial disease can present with rapid infantile liver failure. Two sibling pairs with variants in QIL1, a gene important for mitochondrial contact site and cristae organizing system (MICOS) function, were recently reported on. They had intermittent liver disease, mild cardiac hypertrophy, cerebellar atrophy, acquired microcephaly, neurological impairment, and death before age 5 (12 months to 5 years). Patients also had lactic acidosis and urinary excretion of 3-methylglutaconic acid (3MCGA).(1,2) An additional case had renal stones, liver failure, and progressive neurological decline with death at 22 months.(3) We discuss 7 unreported patients.
Clinical History
Patients 1 and 2 were monozygotic twin sisters who presented with fulminant liver failure at 3 months (Table 1). Electron microscopy (EM) demonstrated unusual cristae configurations in hepatocytes and Kupffer cells, but subtle abnormalities in skeletal muscle mitochondria initially interpreted as normal (Fig. 1). Their mild neurological findings were attributed to liver disease, so they were listed for transplant but died of liver failure. A younger sister (patient 3) had elevated liver function studies at birth. At 9 months, her liver disease was stable. She died at 13 months. Patient 4 had neonatal onset of transient liver disease with death at 10 months from acute respiratory distress syndrome (ARDS). His older sister (patient 5) exhibited a similar course. Liver histology showed enlarged mitochondria (Fig. 1). A sister of the father (patient 6) had a similar course 24 years earlier. Patient 7 had neonatal onset of transient liver failure. She had bouts of liver disease with severe diarrhea and neurological deterioration and died at age 3 in the course of acute liver disease with vomiting and seizures.
TABLE 1.
Key Clinical Features of 7 Patients From Three Families With Variants in QIL1
| Patients | Birth Parameters |
Presentation | Laboratory Findings | Medical Course |
|---|---|---|---|---|
| 1 (Family 1) Female Hispanic d. 4 mo Genetic variant: c.260del (p.Gly87Alafs*3) |
Gestation: 34 weeks Weight: 1,800 g Monozygotic twins |
Month 2: elevated liver enzymes | Month 2: ALT 131 (12-49 u/L); AST 227 (20-98 u/L); total Bili 4.2 (0.1-10.3 mg/dL); direct Bili 3.6 (0.0-0.4 mg/dL) Month 3: ALT 1,206; AST 2,153; ALP: 923 (81-316 u/L); GGT 88 (7-168 u/L); total Bili 12.4; ammonia <10 (<49 μmol/L); lactic acid: 2.7 (1.0-3.5 mmol/L); INR: 2.5; fibrinogen: 96 (200-400 mg/dL); factor V: 27.04 (50%-150%) Urine 3MCGA |
Day 1: poor feeding; 17-day NICU stay Month 1: newborn screen with elevated tyrosine, repeat with methionine elevated Month 2: elevated liver enzymes without jaundice or coagulopathy Month 3: Jaundice with worsening liver enzymes and coagulopathy developing into ALF; poor feeding; brain MRI with normal spectroscopy Month 4: decompensated quickly in the OR with anesthesia and line placement; liver transplant aborted and returned intubated; died 10 days later at 4 months old with multiorgan failure |
| 2 (Family 1) Female Hispanic d. 4 mo Genetic variant: c.260del (p.Gly87Alafs*3) |
Gestation: 34 weeks Weight: 1,990 g Monozygotic twins |
Month 2: elevated liver enzymes | Month 2: ALT 229 (12-49 u/L); AST 296 (20-98 u/L);total Bili 3.3 (0.1-10.3 mg/dL); direct Bili: 2.7 (0.0-0.4 mg/dL) Month 3: ALT 829; AST 1,372; ALP 974 (81-316 u/L); GGT 80 (7-168 u/L); total Bili 7.5; ammonia <10 (<49 μmol/L); lactic acid 4.4 (1.0-3.5 mmol/L); INR: 2; fibrinogen 129 (200-400 mg/dL); factor V 30.02 (50%-150%) Urine 3MCGA |
Day 1: poor feeding; 17-day NICU stay Month 1: newborn screen with elevated tyrosine, repeat with methionine elevated Month 2: elevated liver enzymes without jaundice or coagulopathy Month 3: jaundice with worsening liver enzymes and coagulopathy developing into ALF; poor feeding; liver biopsy with subacute massive hepatic necrosis with collapse, prominent giant cell transformation of residual parenchyma with early micronodular cirrhosis; normal muscle and skin biopsy and brain MRI spectroscopy Month 4: unlisted for liver transplant given biopsy and sister’s clinical course; died at 4 months with worsening coagulopathy |
| 3 (Family 1) Female Hispanic d. 13 mo Genetic variant: c.260del (p.Gly87Alafs*3) |
Gestation: 40 weeks Weight: 3,005 g Length: 50.8 cm APGAR: 9/10 |
Day 1: poor feeding, hypoglycemia, hypothermia; ALF with elevated liver enzymes on day 2 | Day 2: low glucose 40 (47-110 mg/dL); ALT 85 (12-49 u/L); AST 332 (20-98 u/L); ALP 438 (81-316 u/L); GGT 598 (7-168 u/L); total Bili 10.7 (0.1-10.3 mg/dL); direct Bili 0.6 (0.0-0.4 mg/dL); ammonia 60 (<49 μmol/L); lactic acid 8.6 (1.0-3.5 mmol/L); PT 29.1 (9.6-11.6 seconds); INR 2.94; fibrinogen <70 (200-400 mg/dL); Factor V 22.57 (50%-150%) Urine 3MCGA |
Day 1: poor feeding and hypoglycemia requiring gavage feeds and intravenous fluids Day 2: elevated liver enzymes and coagulopathy with ALF Week 1: brain MRI with subtle bilateral cerebral white matter signal abnormality and slight broadening/undersulcation of the frontal lobe gyri Week 3: severe obstructive sleep apnea with associated hypoxemia and oxygen at night; coagulopathy normalized; home with palliative care avoidance of surgery or anesthesia Month 9: all oral feeds, developmental delays Died at 13 months |
| 4 (Family 2) Male Tunisian d. 10 mo Genetic variant: c.143dupT (p.Ala51Argfs*32) |
Gestation: 40 weeks Weight: 2,840 g Length: 47 cm HC: 35 cm APGAR: 10/10 |
Day 3: hypoglycemia, ALF | Day 3: low glucose 29 (47-110 mg/dL); ALT 742 (5-110 u/L); AST 365 (5-55 u/L); ALP 653 (117-270 u/L); AFP 50,480 (150-15,000 ng/mL); GGT 445 (10-270 u/L); total Bili 18.5 (0.1-10.3 mg/dL); direct Bili 2.2 (0.0-0.4 mg/dL); ammonia 67 (<50 μmol/L); lactic acid 6 (1.5-2.0 mmol/L); PT ratio: 23% (70%-100%); factor V 27 (50-150%) Day 6: ALT 120; AST 76; ALP 802; GGT 276; total Bili 7.5; direct Bili 3.5; lactic acid 1.2; normal PT and factor V Month 9: ALT 52; AST 122; ALP 391; GGT 317; normal Bili, PT, and factor V Urine 3MCGA |
Month 6: mild elevation of liver enzymes with normal coagulation profile, mild cholestasis, mild hypotonia; microcephaly (−2 SDs) Month 9: respiratory insufficiency and ARDS Died at 10 months from ARDS |
| 5 (Family 2) Female Tunisian d. 5 mo Genetic variant: c.143dupT (p.Ala51Argfs*32) |
Gestation: 40 weeks Weight: 2,820 g Length: 46.5 cm HC: 34 cm APGAR: 10/10 |
Month 1.5: cyanosis | 1.5 mo: ALT 109 (5-110 u/L); AST 190 (5-55 u/L); ALP 630 (117-270 u/L); GGT 237 (10-270 u/L); normal total Bili; PT ratio 100% (70%-100%) Month 2: ALT 152; AST 165; ALP 458; GGT 297; AFP 1,990 (150-15,000 ng/mL) Month 4: ALT 147 IU/L; AST 78 IU/L; GGT 490 IU/L; ALP 433 IU/L; lactic acid 2-4 (1.5-2.0 mmol/L); no lactaturia; CSF lactate 2.2 (<2 mmol/L); PT ratio 100% Urine 3MCGA |
Month 2: respiratory distress and hepatomegaly Month 4: feeding difficulty with psychomotor regression and microcephaly (HC −2 SDs); breathing difficulties leading to ARDS Died at 5 months from ARDS |
| 6 (Family 2) Female Tunisian d. 9.5 mo Genetic variant: c.143dupT (p.Ala51Argfs*32) |
Gestation: 39 weeks Weight: 3,270 g APGAR: 10/10 |
Month 8: psychomotor regression, microcephaly | Month 8: ALT 537 (5-110 u/L); AST 418 (5-55 u/L); ALP 760 (117-270 u/L); total Bili 3.5 (0.1-10.3 mg/dL); direct Bili 1.8 (0.0-0.4 mg/dL); lactic acid 2 (1.5-2.0 mmol/L); CSF lactate 2.5 (<2 mmol/L); PT ratio 100% (70%-100%) Urine 3MCGA |
Month 8: psychomotor regression, microcephaly at −2.5 SDs, optic atrophy, white matter changes, and cerebellar atrophy; muscle biopsy showed lipidosis, mitochondrial aggregates, and respiratory chain complexes (II + III) and intravenous deficiencies; in liver: deficiency of respiratory chain complexes (II + III) Died at 9.5 months of age from respiratory distress |
| 7 (Family 3) Female Tunisian d. 3 yo Genetic variant: c.143dupT (p.Ala51Argfs*32) |
Gestation: 41 weeks Weight: 3,440 g Length: 52 cm HC: 35 cm APGAR: 10/10 |
Day 1.5: liver failure, hypotonia | Day 1.5: glucose very low; liver enzymes not done; AFP 20,000-50,000 (150-15,000 ng/mL); lactic acid 5 (1.5-2.0 mmol/L); PT ratio 29% (70%-100%); factor V 28% (50%-150%) Day 10: ALT 30 (5-110 u/L); AST 88 (5-55 u/L); GGT 237 (10-270 u/L); ALP 392 (117-270 u/L); total Bili 4.6 (0-15 mg/dL); direct Bili 2.3 (0.0-0.7 mg/dL); lactic acid 5 mmol/L; ammonia 10 (<50 μmol/L); PT ratio 80%; factor V 183% 2 yo 10 mo: ALT 1,440; AST 999; GGT 85; PT ratio 50%; factor V 66%; no metabolic acidosis Urine 3MCGA |
Day 10: liver biopsy with multiple respiratory chain complexes deficiency and mtDNA depletion Month 2: psychomotor delay with cerebellar and optic atrophy on brain MRI Intermittent liver failure when febrile 2 years 10 months: fever, vomiting, neurological deterioration, and associated seizures Died at age 3 from severe neurological deterioration and liver disease |
All families are of consanguinous descent. The proband in each familiy was identified on exome sequencing or mitochondrial nuclear gene panel by next-generation sequencing (patients 4-6) with Sanger confirmation for the proband and all affected relatives.
Abbreviations: AFP, alpha-fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APGAR, Appearance, Pulse, Grimace, Activity, and Respiration; AST, aspartate aminotransferase; CSF, cerebrospinal fluid; d., died; direct Bili, direct bilirubin; HC, head circumference; INR, international normalized ratio; mo, months; MRI, magnetic resonance imaging; mtDNA, mitochondrial DNA; NICU, neonatal intensive care unit; OR, operating room; PT, prothrombin; total Bili, total bilirubin; yo, years old.
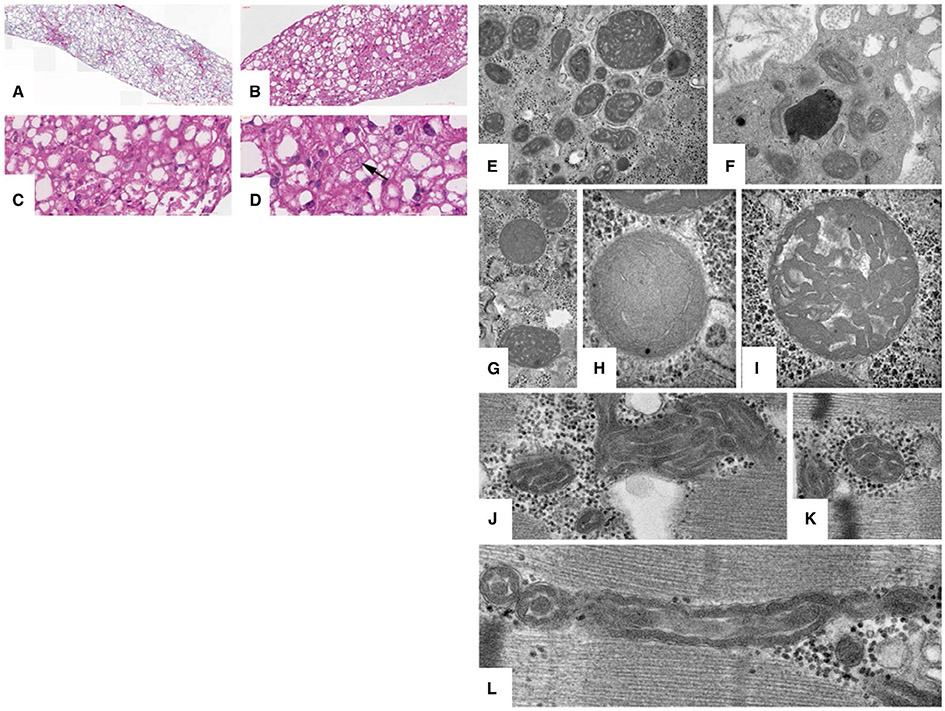
FIG. 1.
Histopathology and ultrastructure of patients 2 and 5. Liver histology on patient 5 (A-D). (A) Liver parenchyma with irregular portal fibrosis and perisinusoidal fibrosis at 25×. (B) Abnormal hepatocytes with diffuse macro- and microvesicular steatosis at 200×. (C) Oxyphilic transformation at 400×. (D) Megamitochondria (denoted with arrow) at 800×. EM images of patient 2 (E-L). (E-I) Pleomorphic mitochondria in hepatocytes exhibit abnormal size variation, with the larger mitochondria exhibiting unusual dysmorphic convoluted stacked cristae resembling a ball of yarn. Intermixed are a few almost normal mitochondria. In all panels, matrix granules are abnormally reduced. (F) The cristae of Kupffer cell mitochondria are also abnormal to a lesser degree. (J,K) Skeletal muscle mitochondria of the same patient exhibit a subtle abnormality consisting of stacked undulating cristae with reduced matrix. (L) The cristae of the smallest muscle mitochondria differ from normal small mitochondria by unusual wavy undulating contours notable in both cross- and longitudinal profiles.
Discussion
Extrahepatic manifestation of QIL1 hepato-encephalopathy varies, but all 7 patients had liver disease with acute liver failure (ALF) in all but 2 patients (5 and 6). In patients 1 and 2, ALF initially appeared to be isolated prompting evaluation for liver transplantation. However, given the high rate of neurological deterioration, mitochondrial hepatopathy attributed to QIL1 ought to be a contraindication for transplantation. Whereas liver disease was the entry point in all patients, its characteristics and severity varied even within the same family. Patients 3, 4, and 7 exhibited neonatal-onset ALF. At presentation, cholestatic jaundice was present in all but 1 patient (patient 5), and gamma-glutamyl transferase (GGT) was normal in 4 patients (1, 2, 5, and 7) and elevated in the other 3. Ammonia was normal or mildly elevated. Lactic acid ranged from 2.7 to 8.6 mmol/L. All patients exhibited urinary excretion of 3MCGA. Therefore, a consistent QIL1 clinical scenario is of early onset and recurrent liver disease of variable severity, most often with cholestatic jaundice and elevated or normal GGT. In that setting, subsequent extrahepatic findings (neurological disease, optic atrophy, and ARDS) and 3MCGA are highly suggestive of QIL1.
For patients 1 and 2, anesthesia exposure likely contributed to their decline given that their sister survived 13 months without anesthesia. ARDS was striking (patients 4 and 5), along with respiratory failure in the literature.(3) This may reflect poor brainstem function with aspiration or be part of the QIL1 phenotype given that QIL1 is expressed in lung tissue.(4)
Characteristic findings on liver EM helps to differentiate this condition. The stacking dysmorphia of the mitochondrial cristae may allow one to distinguish QIL1-related pathology from other metabolic or mitochondrial (mitochondrial DNA depletion syndromes) conditions. However, EM findings vary in different cell types and can be subtle. Although it is not always routine to attain biopsies, multiple images of muscle and liver tissue should be evaluated when attained.
Ultimately, whole-exome sequencing or next-generation sequencing mitochondrial panel (patients 4-6) identified the causative variants in all three families. Because there are only 5 reported cases, QIL1 is not yet on infantile liver failure gene panels. Based on the clinical and management implications of an early diagnosis, we propose that the evaluation of an infant with ALF should include broader genetic testing, especially in the setting of transplant evaluation.
Acknowledgments
This work was supported by NIH grant T32 DK007727.
Abbreviations:
- 3MCGA
3-methylglutaconic acid
- ALF
acute liver failure
- ARDS
acute respiratory distress syndrome
- EM
electron microscopy
- GGT
gamma-glutamyl transferase.
Footnotes
Potential conflict of interest: Dr. Hopkin consults for, advises for, is on the speakers’ bureau for, and received grants from Sanofi Genzyme and Alexion. He consults for, advises for, and received grants from Amicus. He consults for and received grants from Shire. He consults for Sangamo. He received grants from Protalix and Idorsia. Dr. Miethke consults for Shire.
REFERENCES
- 1).Guarani V, Jardel C, Chrétien D, Lombès A, Bénit P, Labasse C, et al. QIL1 mutation causes MICOS disassembly and early onset fatal mitochondrial encephalopathy with liver disease. eLife 2016;5:e17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Zeharia A, Friedman JR, Tobar A, Saada A, Konen O, Fellig Y, et al. Mitochondrial hepato-encephalopathy due to deficiency of QIL1/MIC13 (C19orf70), a MICOS complex subunit. Eur J Hum Genet 2016;24:1778–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Gödiker J, Grüneberg M, DuChesne I, Reunert J, Rust S, Westermann C, et al. QIL1-dependent assembly of MICOS complex-lethal mutation in C19ORF70 resulting in liver disease and severe neurological retardation. J Hum Genet 2018;63:707–716. [DOI] [PubMed] [Google Scholar]
- 4).The Human Protein Atlas. Gene: C19orf70. Atlas; updated: November 15, 2018. www.proteinatlas.org. Accessed April 10, 2019. [Google Scholar]