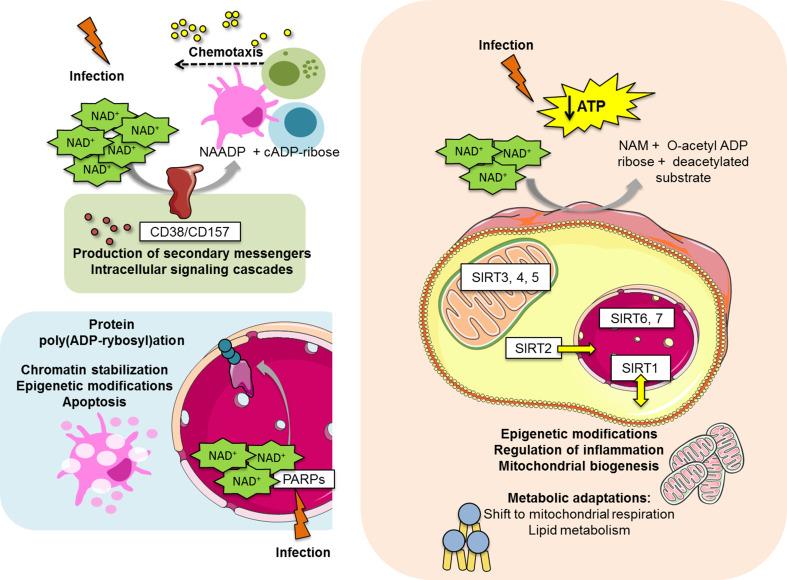
Fig. 2.
Mechanistic action of NAD+-consuming proteins during host–pathogen interaction. The three major classes of NAD+-consuming proteins are involved in NAD+ breakdown and utilization, which originates functional and metabolic alterations upon challenge. NAD+ cleavage in NAADP and cADP-ribose is catalyzed by the ectoenzymes CD38 and CD157. When extracellular NAD+ content is increased, a high NAD+ turnover results in increased concentration of NAADP and cADP-ribose, which contribute for Ca2+ mobilization. This phenomenon leads to the production of secondary messengers, activation of intracellular signaling cascades and consequent chemotaxis of immune cells (for example, dendritic cells, monocytes and neutrophils) towards chemokine gradient. PARPs respond to DNA damage by infectious agents originating poly(ADP-rybosyl)ation of targeted proteins. This may have a role in chromatin stabilization and epigenetic modifications. Furthermore, PARP activation results in NAD+ depletion and cell apoptosis due to decreased energy availability. The seven isoforms of sirtuins are spread in the nucleus (SIRT1, SIRT6 and SIRT7), the cytosol (SIRT2) and the mitochondria (SIRT3, SIRT4 and SIRT5), although SIRT1 may shuttle between the nuclear and cytosolic compartment and SIRT2 was already shown to be able to translocate to the nucleus. Upon sirtuin modulation by the presence of an infectious agent, NAD+ is degraded and ultimately SIRT1 activity originates mitochondrial biogenesis and increased lipid oxidation. These processes lead to an increase of intracellular ATP levels, in an attempt to restore energy homeostasis. Sirtuins and PARPs are also able to cause epigenetic modifications in cellular DNA, which may also contribute to metabolism modulation. ATP adenosine triphosphate, ADP adenosine diphosphate, cADP cyclic adenosine diphosphate, NAADP nicotinic acid adenine dinucleotide phosphate, NAD + nicotinamide adenine dinucleotide, SIRT sirtuin, PARP poly-(ADP-ribose) polymerase