Abstract
Considering the large number of studies focused on myeloid-derived suppressor cells (MDSCs) to date, only a handful of well-defined relationships in human cancer have been established. The difficulty of assessing the impact of MDSCs in human cancer is partly due to the relatively small number of studies performed in humans. This is compounded in the literature by a common lack of clear indication of which species is being referred to for each characteristic described. These aspects may result in inappropriate extrapolation of animal studies to those in the human setting. This is especially the case for studies focused on investigating therapies which can be used to target MDSCs or those aimed at understanding their mechanism. Here, we attempt to rectify this by reviewing only studies on MDSC performed in humans. We survey studies which explore (1) whether MDSC levels are altered in cancer patients and if this is correlated with patient survival, (2) the so far identified mechanisms employed by MDSC to exert immune suppression, and (3) whether therapeutic agents can be used to target MDSCs by either altering their level, influencing their differentiation or inhibiting their suppressive function. Despite the fact that these studies clearly show that MDSCs are important in human cancer, the clinical employment of agents intended to target them has not yet been accomplished. We identify factors which have contributed to this and propose steps which may facilitate the translation of these therapies to the clinic in future.
Keywords: MDSCs, Clinical, Targeting, Suppression, Mechanism, Human
Introduction
Myeloid cells mature from hematopoietic stem cells (HSC) into terminally differentiated populations. These include dendritic cells (DCs), macrophages and granulocytes. However, chronic inflammation in cancer can result in the presence of secreted factors that prevent or disrupt this maturation process, thereby generating populations of immature cells at different stages of differentiation, of which some may be immunosuppressive (reviewed in [1, 2]). To emphasise their suppressive nature, these cells have been termed myeloid-derived suppressor cells (MDSCs) [3]. These cells have been shown to be associated with tumour progression and have consequently been gaining attention in the field of cancer research. MDSCs were originally described in tumour-bearing mice where they can be identified solely by the expression of CD11b and Gr-1 [1, 4]. These are currently organised into two main populations using expression of the Gr-1 isoforms Ly6G and Ly6C. Granulocytic MDSCs (grMDSCs) are defined as CD11b+Ly6C−Ly6Ghigh and monocytic MDSCs (moMDSCs) as CD11b+Ly6ChighLy6G−(reviewed in [5, 6]. However, there is no Gr-1 homologue in humans and a universal and accurate definition of MDSCs using other markers has not yet been achieved. Because of this, studies have investigated multiple different candidate molecules in an attempt to identify specific markers for human MDSC [7]. This has resulted in a large number of different phenotypes being reported. General consensus across current studies has allocated human MDSC into three predominant populations. MoMDSCs which morphologically resemble monocytes and express CD14 but usually not CD15 [8, 9]. GrMDSCs share morphological characteristics with granulocytes and express CD15 and/or CD66b but lack CD14 [9–12]. Immature MDSCs represent the least well-defined population and lack expression of CD14 or CD15 [13, 14].
Detailed information on the origin, differentiation and development of MDSCs has been extensively covered in other publications [6, 7, 15] and is only outlined in brief here. In healthy individuals, bone marrow-generated immature myeloid cells (IMCs) develop from common myeloid progenitor cells which differentiate into mature non-suppressive myeloid cells including, DCs, macrophages or granulocytes after entering the blood stream and migrating to tissue-specific sites. Factors produced in response to infection, inflammation or by tumour cells can direct the differentiation of IMCs away from mature non-suppressive cells into those with suppressive function. This disrupted maturation can lead to the activation and accumulation of suppressive myeloid cells that contribute to local and systemic immune suppression. Several secreted factors have been reported to be involved in the expansion and activation of MDSCs. These can either be secreted by tumour cells themselves, or by tumour stromal cells, such as activated T cells. COX-2 and PGE2 [8, 16–18] can both stimulate myelopoiesis, inhibit the differentiation of IMCs into mature myeloid cells and lead to the accumulation or activation of MDSCs. Low dose GM-CSF can have immune stimulatory functions [19] while higher levels have been shown to expand populations of myeloid cells with suppressive properties [8, 13, 20, 21]. Secreted levels of IL-6 [21, 22], IL-10 [22] IL-13 [23] and TNF [21, 24] were also correlated with the presence of different MDSC subtypes. Direct evidence of tumour-specific regulation of MDSC differentiation in humans comes from in vitro studies which have shown that inflammatory factors produced by tumour cells direct their differentiation into cells with suppressive ability [17, 24]. These results suggest that the programming of immune suppression occurs in the tumour microenvironment in situ.
Considering the amount of studies to date, it is noteworthy that only a handful of well-defined relationships in human cancer have been established. One of the major issues contributing to the lack of clarity is the large number of studies which have been performed using animal models, resulting in the unwarranted extrapolation of results from animals to those in humans. Caution should be exercised due to the demonstrated biological differences between the murine and human immune systems [25]. This ongoing issue can be confounded by the lack of clear species indication in the literature when discussing, reporting and interpreting experimental results, especially when referring to the work performed by other investigators. This is particularly problematic in the clinical setting where our view of MDSCs, especially of the therapeutic strategies aimed at targeting them, is largely derived from animal studies. In humans there are currently no clinically available agents which specifically act on MDSCs, but they have been successfully targeted in mice to result in prolonged survival [26].
In this review, we attempt to rectify this by limiting our focus to studies which have solely investigated MDSCs in humans. The scope of this review branches across three themes where we survey studies that have (1) compared MDSC levels between cancer patients and healthy individuals and examined MDSCs in association with clinical response to therapy and tumour burden, (2) examined the mechanisms employed by MDSC to suppress the immune system, and (3) investigated therapeutic strategies that can be used to target MDSCs.
Clinical relevance of MDSCs in human cancer
This section focuses on MDSC populations which have been shown to be clinically relevant in human cancer to date. Considering the multiple MDSC phenotypes that have been reported, this section is broadly organised according to the three major groups of MDSC phenotypes: (1) moMDSC which express CD14, but typically not CD15, (2) grMDSC which show expression of CD15 or CD66b but lack CD14, and (3) immature or undefined-phenotypes which are negative for CD14 and/or CD15 or have not been defined according to CD14, CD15 or CD66b. The majority of studies have been performed using peripheral blood, but a small number also investigated MDSCs in tissue. These studies investigating the clinical relevance of MDSCs can be broadly grouped into the following categories: those comparing the level of MDSCs between cancer patients and healthy individuals (“MDSC levels in human cancer and response to therapy”); those which have investigated MDSC levels in relation to tumour burden and clinical stage (“MDSC levels in human cancer and response to therapy”); reports showing that MDSC levels relate to patient response to cancer therapies (“MDSC levels in human cancer and response to therapy”), and MDSC levels in association with patient survival (“MDSC levels in association with patient survival”).
MDSC levels in human cancer and response to therapy
The most common moMDSC phenotype reported to be associated with clinical features in cancer patients is CD14+HLA-DR−/low (studies describing this phenotype may have also used other markers, such as CD11b, CD33 and different lineage cocktails, but this phenotype will be referred to herein as “CD14+HLA-DR−/low”). Twenty-two studies reported significantly more CD14+HLA-DR−/low MDSCs compared with healthy individuals in the peripheral blood of cancer patients including melanoma [8, 27–34], prostate cancer [35, 36], bladder cancer [37], hepatocellular carcinoma (HCC) [38, 39], non-small cell lung cancer (NSCLC) [40, 41], chronic lymphocytic leukaemia (CLL) [42], esophageal squamous cell carcinoma (ESCC) [43, 44], Hodgkin lymphoma [45], renal cell carcinoma (RCC) [46], and one study showing higher levels in head and neck squamous cell carcinoma (HNSCC) patients in comparison with chronic inflammatory disease patients [47]. A study by Gros et al. [30] was noteworthy in finding that of 14 phenotypically defined circulating MDSC populations, the percentage of HLA-DR−cells within the CD14+ population was the only one observed to be higher in melanoma patients than healthy donors. The majority of these studies also reported that the CD14+HLA-DR−/low MDSC population was associated with clinical parameters, such as tumour progression, cancer pathological grade and clinical stage [27, 28, 33, 35–38, 40, 47–49] in different types of cancer. Another point worth considering when comparing MDSC levels between patients and controls is that levels of monocytes themselves have also been shown to differ between cancer patients and healthy individuals [50].
In contrast to the commonly reported influence of circulating moMDSCs on clinical parameters, less evidence is available for grMDSCs in human cancer. We identified twelve studies performed in patients with NSCLC [41, 49], HNSCC [51], bladder cancer [52], prostate cancer [53], HCC [38], melanoma [32], pancreatic cancer [54], gastric cancer [55], RCC [46], lung cancer, breast cancer, gastrointestinal cancer and others [50, 56], which reported significantly higher levels of grMDSCs in cancer patients compared with healthy individuals, or discussed their association with clinical parameters, such as stage of disease. Notably, the phenotype CD15+CD14−CD33+CD11b+ investigated by Liu et al. [49] resulted in very robust relationships, perhaps due to the significantly larger cohort examined in this study. This provides convincing evidence for the clinical relevance of grMDSCs in addition to the more widely studied moMDSCs.
The remainder (12) of the identified studies was performed either on less well-defined MDSC phenotypes (those which might express mo- or gr-markers but were not tested) or on immature/undifferentiated phenotypes (those that were negative for mo- and gr-markers). A recent study by Wu et al. [57] for example, revealed that the frequencies of certain circulating granulocyte-monocyte progenitors (GMPs) were markedly higher in the peripheral blood of seven different cancer patient cohorts (HCC, breast cancer, gastrointestinal cancer, ovarian cancer, lung cancer, esophageal cancer, and cervical cancer) compared to healthy donors. The authors also showed that MDSC levels correlated with clinical stages in HCC, cervical and gastrointestinal cancer. Significantly more MDSCs compared with healthy individuals as well as clinical correlations were also found in further studies including prostate cancer [58], Hodgkin lymphoma [45], melanoma [28, 59], breast cancer [14, 60], colorectal cancer [61, 62], RCC [46], pancreatic cancer, esophageal and gastric cancer [23] and other cancer types [50].
Further emphasising the clinical importance of MDSC populations in blood, their levels have been shown to be associated with clinical response to cancer treatments, rendering patients with high MDSC levels less sensitive to hormone [35], chemo- [13, 40, 41, 43, 63], radio- [50, 64, 65] and immunotherapy [66, 77]. Despite these studies showing that MDSC levels relate to cancer therapy, we identified few compared with those showing that MDSCs are associated with tumour burden and patient survival. Thus, this area appears to be relatively understudied, but a better understanding of how MDSC levels relate to patient response to therapy is vital to improving cancer treatment.
It is noteworthy that many studies which report MDSCs to be clinically relevant in blood also performed functional assays demonstrating that the cells in question exerted suppressive effects on autologous T cells, thereby suggesting a causal link between their presence and the clinical outcome. Some studies document the suppressive effects of MDSCs in HCC [38, 39], melanoma [8, 27, 28, 30–32, 67], prostate cancer [35, 36, 53, 58], bladder cancer [37, 52], HNSCC [47] and NSCLC [40, 49], breast cancer [14] gastric cancer [55], colorectal cancer [61] and others [50, 56, 57]. Unlike for phenotypic analysis, these studies only tested small numbers of samples (typically around 5, but ranging from 2 to 16) when investigating suppressive ability. The methods used to assess suppression also differed, for example, isolated MDSCs were cultured with stimulated T cells in some studies, but others merely depleted myeloid cells or MDSCs from stimulated PMBCs. These approaches do not provide an equal degree of specificity as to the reported effect. Standardisation of testing conditions may facilitate more effective comparison of results from different studies in future. Despite this, the published results collectively indicate that MDSCs are suppressive in the same patient cohorts also showing that these cells are clinically relevant.
While the majority of studies on human MDSC focus on these cells in the periphery, few have examined their role in tumour tissue. The majority of the latter few studies were performed with low sample numbers that limit the determination of their clinical significance. The tissue levels of CD14+HLA-DR−/low moMDSC were investigated in HCC [39] and HNSCC [47] where there was a relatively high abundance of these cells in the examined tumours. CD14+HLA-DR−/low MDSCs from HNSCC tissue correlated with cancer stage and suppressed autologous T cell proliferation, similar to those from blood [47]. Gros et al. [30] revealed that CD14+ cells were the most predominant myeloid subset in melanoma tumour tissue, but it is noteworthy that these cells displayed higher expression of HLA-DR and showed an impaired ability to inhibit T cell proliferation by comparison with those in the periphery. Different investigators have also observed a high level of infiltration by several grMDSC subsets in pancreatic [54], bladder [52], prostate [53] and colon tumours [57]. In what may be the largest study investigating tissue MDSCs, HLA-DR−CD33+ cells in the tumours of colorectal cancer patients were found to correlate with metastatic spread and tumour stage [62]. Immature myeloid precursor cells were also found to accumulate in colon cancer [57], ovarian cancer [68] and in breast cancer where they correlated with clinical stage and lymph node metastasis [60]. However, it should be pointed out that only four of all identified studies investigating MDSCs in tumour tissue (n = 11) compared this with the level in corresponding benign tissue controls [54, 57, 60, 61]. More commonly, MDSC levels in tissue were compared with those in blood of the same patient or blood of healthy individuals, or levels in tissue were compared across patients with a different disease stage or type. Thus, most studies investigating tissue MDSCs lack appropriate matching benign controls, unlike the study of MDSCs in blood. A further limitation of these studies is that the investigation of the suppressive capacity of tissue-infiltrating MDSCs is restricted to four of the eleven studies which examined tissue MDSCs in relation to clinical parameters or in comparison to healthy individuals [30, 47, 60, 68]. This leaves open the possibility that some of these authors investigated non-MDSC myeloid cells, a point worth considering given that mere phenotypic characterisation is not necessarily sufficient to distinguish between MDSCs and other non-suppressive myeloid cells.
MDSC levels in association with patient survival
As illustrated in the previous section, elevated levels of MDSCs in different types of cancer and corresponding correlations with disease stage or tumour burden have frequently been reported. In contrast, studies showing a correlation of MDSC levels and clinical outcome have been relatively scarce. Because there is comparatively little literature regarding the association of MDSC on survival in human cancer, this section summarises publications on this topic (Table 1).
Table 1.
MDSC levels in association with patient survival
| Phenotype | Cancer type | Patient number | Threshold | PT | Treatment after blood draw | Reference population | Refs. |
|---|---|---|---|---|---|---|---|
| Monocytic MDSCs | |||||||
| CD14+HLA-DR−CD15+CD33+CD11b+Lin− | NSCLC | N = 104 (p = 0.0082) | 2.2 % | No | Chemotherapy (±Bevacizumab) | CD11b+CD14+ cells | [41] |
| CD14+HLA-DR−/lowCD15−CD33+CD11b+Lin− | Prostate cancer | N = 41 (p = 0.13) | NM | Yes | Mix | Live cells | [36] |
| CD14+HLA-DR−/lowCD15−CD33+CD11b+CD34−CD80−CD83−PD-L1− | NSCLC | N = 60 (p < 0.01) | 9.43 % or 44.7 cells/µL | No | Chemotherapy | CD14+ cells | [40] |
| CD14+HLA-DR−/lowCD15−CD11b+ | Melanoma | N = 51 (p = 0.007) | 2.3 % | Yes | Mix | Live cells | [29] |
| CD14+HLA-DR−/lowCD11b+Lin− | Melanoma | N = 68 (p = 0.002) | 14.9 | Yes | Ipilimumab | Lin−CD14+CD11b+ cells | [31] |
| N = 133 (p < 0.001) | 11 % | Yes | Mix | Live cells | [69] | ||
| CD14+HLA-DR−/lowCD11b+ | Melanoma | N = 33 (p = 0.08) | 2.9 % | No | Peptide Vaccination | CD14+ cells | [73] |
| CD14+HLA-DR−/lowLin− | Breast cancer | N = 40 (p = 0.03) | NM | No | Mix | CD45+ cells | [71] |
| CLL | N = 49 (p = 0.0001) | 40 % | No | NM | CD14+ cells | [42] | |
| Melanoma | N = 164 (p < 0.001) | 5.1 % | Yes | Ipilimumab | Live cells | [74] | |
| Prostate cancer | N = 24 (p = 0.0046) | 0.3 % | ND | Ipilimumab + GVAX | Live cells | [75] | |
| CD14+HLA-DR−/low | ESCC | N = 76 (p = 0.0036) | 2.38 % | No | None | Single cells | [44] |
| HCC | N = 123 (p = 0.008) | 22 % | No | Radiofrequency ablation | CD14+ cells | [38] | |
| RCC | N = 57 (p = 0.0035) | NM | Yes | Peptide Vaccination | Single cells | [46] | |
| Granulocytic MDSCs | |||||||
| CD15+CD14−HLA-DR−/lowCD33+CD11b+Lin− | Gastric cancer | N = 40 (p = 0.024) | 4 % | No | Chemotherapy | Total cells | [55] |
| CD15+CD16low | Lung, breast, gastrointestinal and other | N = 53 (p = 0.001) | 293 cells/mL | Yes | NM | [56] | |
| Immature/undefined MDSCs | |||||||
| CD33+CD11b+CD14−HLA-DR−Lin− | Melanoma | N = 34 (p = 0.0162) | NM | Yes | NM | Live cells | [28] |
| CD33+CD11b+CD14−HLA-DR− | Prostate cancer | N = 62 (p < 0.001) | NM | No | Chemotherapy | Live cells | [58] |
| CD33+CD11b+HLA-DR−Lin− | Breast cancer | N = 26 (p = 0.048) | 3.17 % | Yes | Chemotherapy | Total cells | [13] |
| Colorectal cancer | N = 25 (p = 0.025) | 2.54 % | Yes | Chemotherapy | Total cells | [13] | |
| Pancreatic, esophageal and gastric cancer | N = 123 (p < 0.001) | 2 % | Yes | Mix | Live cells | [23] | |
| Melanoma | N = 27 (p = 0.03) | NM | No | Ipilimumab | Monocytes | [77] | |
| N = 69 (p = 0.012) | 4.13 % | Yes | NM | Live cells | [59] | ||
| CD33+CD34+CD11b+HLA-DR−CD14− | Hodgkin lymphoma | N = 60 (p < 0.001) | 0.0045 × 109/l | ND | Chemotherapy | CD34+ cells | [45] |
| CD33+CD2−CD19−CD45+Lin− | Ovarian cancer | N = 137 (p = 0.006) | NM | ND | NM | NM | [68] |
CLL chronic lymphocytic leukaemia, ESCC esophageal squamous cell carcinoma, HCC hepatocellular carcinoma, NM not mentioned, NSCLC non-small cell lung cancer, PT pretreatment, RCC renal cell carcinoma
A correlation between high levels of moMDSCs and shorter overall survival (OS) or progression-free survival (PFS), using cryopreserved material, has been observed in different types of cancer including CLL [42], ESCC [48], HCC [38], NSCLC [41], and prostate cancer [36]. An enrichment of moMDSCs correlated with decreased PFS in late-stage melanoma [29], and another study identified their levels as a negative independent prognostic factor [69]. It is noteworthy that in the latter study MDSC levels inversely correlated with the presence of T cell responses to the tumour-associated antigens NY-ESO-1 and Melan-A, which themselves are prognostic factors in melanoma [70]. Similarly, breast cancer patients with low frequencies of moMDSCs and T cells responding to human epidermal growth factor receptor 2 (HER2) peptides in vitro were found to have a significant survival benefit compared to patients with high MDSC levels and a lack of HER2 T cell response [71], suggesting MDSCs as potent suppressors of otherwise beneficial T cell responses. A multi-centre randomised phase II clinical trial conducted by Walter et al. [72] reported a survival disadvantage of high baseline moMDSC levels in RCC patients receiving multi-peptide vaccination. In line with this, a trend towards improved disease-free survival (DFS) was described for melanoma patients treated with HLA class I peptides who had low levels of moMDSCs [73]. High pretreatment levels of moMDSCs were not only informative for peptide vaccination but were also associated with reduced OS in melanoma patients receiving immunotherapy with ipilimumab [31, 74]. Similar results have been obtained in an independent cohort of prostate cancer patients treated with ipilimumab in combination with an allogenic tumour cell based vaccine [75]. Consistent with the findings obtained using cryopreserved material, PFS of patients with NSCLC after cisplatin based chemotherapy was shorter for those with higher frequencies and higher absolute numbers of moMDSCs from fresh peripheral blood samples [40]. Along the same lines, in lung cancer patients vaccinated with telomerase peptides, levels of CD33+MDSCs were inversely associated with the immune response rate [76].
While correlations of levels of several moMDSC populations with OS have frequently been observed there are very few publications describing similar effects for grMDSCs. For example, gastric cancer patients with high levels of grMDSCs were found to have a shorter OS compared with those who had low levels [55]. GrMDSCs were further confirmed as an independent factor with multivariate analysis in this study. Interestingly, administration of chemotherapy was able to reduce grMDSCs predominantly in patients showing an objective clinical response to therapy. Low levels of freshly isolated grMDSCs were associated with prolonged survival in patients with different types of terminal disease, including lung, breast and gastrointestinal cancer [56].
Elevated levels of MDSCs with an immature or undefined phenotype were associated with poor survival and identified as an independent prognostic factor in a large mixed cohort study consisting of patients with pancreatic, esophageal and gastric cancer [23]. This was later confirmed in a study by Chevolet et al., where the same MDSC population was shown to be an independent prognostic factor associated with worse OS but not PFS in patients with different stages of melanoma [59]. A study by Jordan et al. [28] found that frequencies of MDSCs correlated with disease progression and OS in melanoma, with similar results later obtained in prostate cancer [58]. Patients treated with neoadjuvant therapy consisting of ipilimumab following surgery showed a significant decrease in levels of circulating MDSCs. Patients who experienced a greater than median decrease in MDSCs also showed superior PFS [77]. Along these lines, patients with breast and colorectal cancer who had high MDSC levels at the start of a new line of systemic chemotherapy had a worse prognosis than those with lower MDSC levels [13]. The PFS of patients with Hodgkin lymphoma was significantly shorter for those with high circulating levels of a freshly isolated immature MDSC population at diagnosis [45]. Treatment-naïve HCC patients with high levels of GMPs had a reduced time to progression compared to others [57]. Moreover, OS and DFS was longer for ovarian cancer patients with low tumour infiltration by MDSCs, and a strong negative correlation was found between high tumour infiltration by MDSCs and low expression of C-terminal binding protein-2 [68].
We identified twenty-four studies which have shown that MDSC levels are associated with clinical outcome in a wide range of cancers from diverse histological origins. It is noteworthy that these associations are present despite the use of different phenotypic marker combinations, reference populations, thresholds and methods that have been employed to characterise and quantify these cells, underlining their important role in tumour immunity. Despite all these data, a number of issues should be given more attention in future studies. For example, it is as yet not entirely clear if MDSCs are causally linked to the observed survival associations, or merely represent surrogate markers of other parameters. The widely held belief that the specific cancer type is responsible for the expansion of a particular MDSC subpopulation remains controversial because different MDSC populations have been shown to be associated with patient outcome in the same cancer entity.
To conclude this section, there is an ever increasing number of studies which show that MDSC levels are different in cancer patients than controls, relate to patient response to therapy and correlate with patient survival (“MDSC levels in human cancer and response to therapy” and “MDSC levels in association with patient survival”). Despite correlations being reported in a number of different cancer types, there is no universal standard in the definition of MDSC phenotypes. This large number of studies has resulted in a wide range of reported MDSC phenotypes, whereby some have been studied in only one particular cancer type or where multiple studies in the same cancer type have assessed MDSC populations with similar or entirely different phenotypes. This lack of harmonisation remains an ongoing issue because little is known regarding the possible overlap or the extent of such subpopulation overlap between the different phenotypes reported across studies [7]. The first attempts to address this have recently been reported [78], but clearly more focus should be placed on technical aspects of MDSC characterisation. This is an ongoing obstacle in defining the clinical impact of human MDSC. It should also be highlighted that study sizes differ considerably; some have drawn statistically significant conclusions with 20 or fewer patients. This may contribute to the generation of misleading results; studies performed with very low sample sizes of this type should be viewed with a high degree of caution until they are validated. Given the problematic detection of grMDSC in frozen samples [51, 79], this is an aspect that should be considered when assessing their levels in peripheral blood. Finally, there is also an urgent need for markers that assist in the precise identification of MDSCs as being distinct from their non-suppressive counterparts. This is especially true for monocytes, because these have also been reported to show some degree of immune suppression [80] and like MDSCs, to correlate with patient survival in melanoma [81].
Mechanisms employed by MDSC to suppress the immune system
There have been a large number of studies dedicated to understanding the suppressive mechanisms of MDSC, but relatively few of these were performed in humans. To avoid the unjustified translation of results from animal studies to those in human, we assessed studies which have described mechanisms by which human MDSCs suppress the immune system. MDSCs have been found to employ a range of different cellular and molecular suppressive strategies. These cells are capable of inducing the differentiation of suppressive regulatory T cells (Tregs) which in turn exert their own type of immune suppression [82], depleting arginine required for the activation of T cells [83], generating reactive oxygen species (ROS) which can damage cells and interfere with cellular functioning [84], releasing suppressive soluble molecules, such as transforming growth factor-β (TGF-β) [85] and prostaglandin E2 (PGE2) [86], or through other less well-defined pleiotropic pathways, such as through the signal transducer and activator of transcription-3 (STAT3) transcription factor [87].
We identified 18 studies performed in humans in at least ten different cancer entities that reported different mechanisms by which MDSCs are able to suppress the immune system (Table 2). By far the most common measure of immune suppression was T cell stimulation, with the effect on natural killer (NK) cells and dendritic cells (DCs) also rarely assessed. Even though MDSCs are claimed to be heterogeneous in many respects, a relatively narrow set of suppressive mechanisms has been defined so far in human MDSCs. The most commonly reported mechanisms in these cancers involve Treg induction [39, 88], ROS [10, 17, 89], arginase [33, 47, 53, 90], TGF-β [8, 16, 91] and the overlapping PGE2/COX-2/STAT3 pathways [17, 33, 47, 53, 60]. Despite this, it is worth pointing out that different suppressive mechanisms have been reported in the same cancer type. For example, Filipazzi et al. [8] reported a suppressive mechanism mediated by TGF-β but not arginase by moMDSC in melanoma, whereas Poschke et al. [33] showed that in their melanoma patients, moMDSC suppressed through arginase but with only weak or inconsistent results for TGF-β. This could be due to these studies using different sample types, with the former using peripheral blood mononuclear cells (PBMC) from post vaccinated patients. Alternately, these differences may indicate that within the same cancer type, MDSC employ mutually exclusive mechanisms to suppress the immune system. Conversely, most of these studies were able to identify an immune suppressive effect and attribute it to a certain pathway, but few were able to show that inhibition of this pathway fully reversed MDSC-mediated suppression. This suggests that in addition to non-redundant suppressive pathways in different groups of patients in the same cancer type, multiple suppressive mechanisms can be simultaneously employed by MDSC, each contributing in a redundant or independent way to suppress the immune system. Indeed, some of these studies showed that inhibiting multiple different suppressive pathways each resulted in a reduction of suppressive capacity [17, 24, 91].
Table 2.
Mechanisms employed by MDSC to suppress the immune system
| Suppressive mechanism | Phenotype in functional testing | Suppression assessment | Cancer, sample type | Refs. |
|---|---|---|---|---|
| pSTAT3 via ARG (Tissue and blood MDSC) | CD14+HLA-DR−/low* | T cell stimulation | HNSCC, PBMC and tumour | [47] |
| STAT3 via ARG | CD15+CD14−* | T cell stimulation | Prostate cancer, PBMC | [53] |
| ARGa (restored proliferation), STAT3 (restored proliferation and IFN production) (weak or inconsistent results for iNOS, TGF-β). No effect for CD80, CD83 or DC-SIGN | PBMC or CD14−depleted PBMC | T cell stimulation | Metastatic melanoma, PBMC | [33] |
|
COX-2 (trend for STAT3, weak or inconsistent results for ROS, iNOS, ARG, TGF-β) (in vitro generated MDSC) STAT3, PGE2, ROS (trends for COX-2, CD80, TGF-β, ARG, adenosine) (Patient MDSC) |
CD14+* | T cell stimulation | Metastatic melanoma and healthy donor PBMC with melanoma cell lines | [17] |
|
IDO/T cell apoptosis (MDSC from tissue) IDO/pSTAT3/T cell apoptosis (in vitro generated MDSC) |
CD33+* CD45+CD33+CD13+CD14−CD15−* |
T cell stimulation | Breast cancer tissue and healthy donor UCB | [60] |
| COX-2/PGE2 (involvement of ARG, ROS) | CD33+* | T cell stimulation | Healthy PBMC with cancer cell lines | [24] |
|
TGF-β/PGE2 (in vitro generated MDSC) TGF-β, ROS (catalase) (no effect for PGE2, SOD, ARG, iNOS) (Patient MDSC) |
CD14+cells* CD14+HLA-DR−/low* |
NK cell cytotoxicity | Metastatic melanoma and healthy PBMC | [16] |
| Partially via ARG, iNOS (no effect for ROS, COX-2) | CD11b+CD14−HLA-DR−/lowCD33+CD15+* | T cell stimulation | Bone marrow from relapsed multiple myeloma, PBMC | [90] |
| Depletion of CD11b+(CD11b+CD14- high ARG activity) but not CD14+(CD11b+CD14+ no ARG activity) restored T cell stimulation. (IDO not detected, CD14+ and CD15+ cells produced similar levels of H2O2) | PBMC or CD11b or CD14 depleted PBMC | T cell stimulation | RCC, PBMC | [11] |
| TGF-β (no effect for ARG and iNOS) | CD11b+CD14+HLA-DR−/low* | Lymphocyte stimulation | Post vaccinated melanoma, PBMC | [8] |
| Partially via CD86, PD-L1, TGF-β | CD14+HLA-DR−/low* | T cell stimulation | HNSCC, PBMC | [91] |
| ROS | CD15+* | T cell stimulation | Healthy PBMC (activated granulocytes) | [10] |
|
IDO/Treg inductionb (no effect for PD-1 or HLA-G) (in vitro generated and patient MDSC) |
CD14+HLA-DRlow* | T cell stimulation | Untreated CLL and healthy donor PBMC with CLL cell lines | [88] |
| Treg induction (Conversion of CD4+IL-17+ cells into IL-17+FOXP3+ and IL-17−FOXP3+ cells (involvement of ATRA and TGF-β) and CD4+ (CD45RA+) into CD4+FOXP3+ (involvement of ATRA and TGF-β, no effect for RARγ, IL-1ß, IL-6 or TNF-α) | CD14+HLA-DR−/low* | No functional testing; phenotype only | PBMC (unspecified origin) | [117] |
| CD15+ but not CD14+ depletion improved T cell proliferation and resulted in less Treg induction (CD4+FOXP3+) with PMA/Ionomycin stimulation | PBMC or CD15 depleted PBMC | T cell stimulation; phenotype | Superficial noninvasive and invasive UC, PBMC | [52] |
| Suppression of NK cell cytotoxicity via NKp30a. (No effect for IDO, ARG, iNOS, NKG2D, CD94, NKp44, MHC-I, HLA-DR, pSTAT1) | CD14+HLA-DR−/low* | NK cell cytotoxicity | HCC, PBMC | [118] |
| DC suppression (impaired maturation, reduced antigen uptake, reduced migration, impaired T cell stimulation and altered cytokine production) | CD14+HLA-DR−* | DC function | Melanoma, PBMC | [119] |
| ARG, suppression of peptide-reactive T cells, inability for DC differentiation, inability to stimulate allogenic leukocytes, induction of Treg phenotypes CD4+FOXP3+a (no effect for ARG) and CD4+FOXP3− (IL-10) | CD14+HLA-DR−/low* | Lymphocyte stimulation, DC generation, T cell stimulation | HCC, PBMC | [39] |
ARG arginase, ATRA all-transretinoic acid, CLL chronic lymphocytic leukaemia, COX-2 cyclooxygenase-2, DC dendritic cell, HCC hepatocellular carcinoma, HNSCC head and neck squamous cell carcinoma, IDO indoleamine 2,3-dioxygenase, iNOS inducible nitric oxide synthase, NK natural killer cell, PBMC peripheral blood mononuclear cells, PD-L1 programmed death-ligand 1, PGE2 prostaglandin E2, PMA phorbol 12-myristate 13-acetate, RARγ retinoic acid receptor gamma, RCC renal cell carcinoma, ROS reactive oxygen species, SOD superoxide dismutase, STAT3 signal transducer and activator of transcription 3, TGF-β transforming growth factor beta, TNF-α Tumour necrosis factor alpha, Treg regulatory T cell, UC urothelial carcinoma, UCB umbilical cord blood
* Isolated cells
a Cell contact dependent
b Cell contact independent
One of the major limitations of this work taken as a whole is the narrow assessment of immune suppression, with the majority of studies only examining suppressive effects against non-specifically stimulated T cells. This form of artificial stimulation that does not occur in vivo may not be relevant to T cell suppression mediated in vivo. A more diverse assessment of how these MDSCs exert immune suppression may lead to a better understanding of how they function in in vivo; it has not yet been examined if MDSCs which suppress through a particular mechanism are generally immunosuppressive or if they preferentially suppress certain immune functions while leaving others unaffected. For example, it is not known if MDSCs are suppressive against a range of different immune processes (DC maturation, T cell proliferation, NK cell cytotoxicity, through the induction of Tregs, etc.) or if they show selective suppression against specific immune functions. A further limitation of this body of work is that the majority of studies to date has investigated moMDSCs in peripheral blood; a mechanistic understanding of grMDSC in blood and MDSC in tissue remains relatively understudied. Even within the most frequently studied moMDSCs, it is difficult to directly compare the details of these studies; some studies used in vitro generated MDSC while others used those from patients ex vivo. Furthermore, they were performed under different testing conditions (differences in sample origin or cancer type, reagents, cell ratios used in suppression assays, and differences in how suppression was measured for example T cell proliferation vs. T cell cytokine production). Despite this, it is clear that MDSCs can be suppressive to the immune system and that they achieve this through a handful of so far identified mechanisms. Future studies should be more orientated towards attempting to uncover the full suppressive repertoire in terms of the different immune processes that are affected and the mechanisms through which this is achieved.
Therapeutic strategies to target MDSCs
Because MDSCs are associated with clinical features in cancer patients, targeting these cells is viewed as a promising therapeutic approach in the treatment of human cancer. Given the complex network of signalling molecules involved in the differentiation, expansion, recruitment and immunosuppressive mechanisms of MDSCs, a range of different therapeutic strategies is required to target them, with the ultimate goal of boosting anti-tumour immunity (Fig. 1). Strategies for overcoming MDSC-mediated immune suppression have so far focused on three broad approaches: (1) reducing their level, (2) inhibiting their suppressive function, or (3) influencing their differentiation. Tested approaches include the use of less specific agents such as vitamin A (ATRA), vaccines (heat shock protein-based), or more specific approaches such as immunotherapies (ipilimumab) and targeted drugs (sunitinib, vemurafenib and bardoxolone methyl (CDDO-Me)). See Table 3 for a full list of therapeutic interventions used to target MDSCs in humans to date.
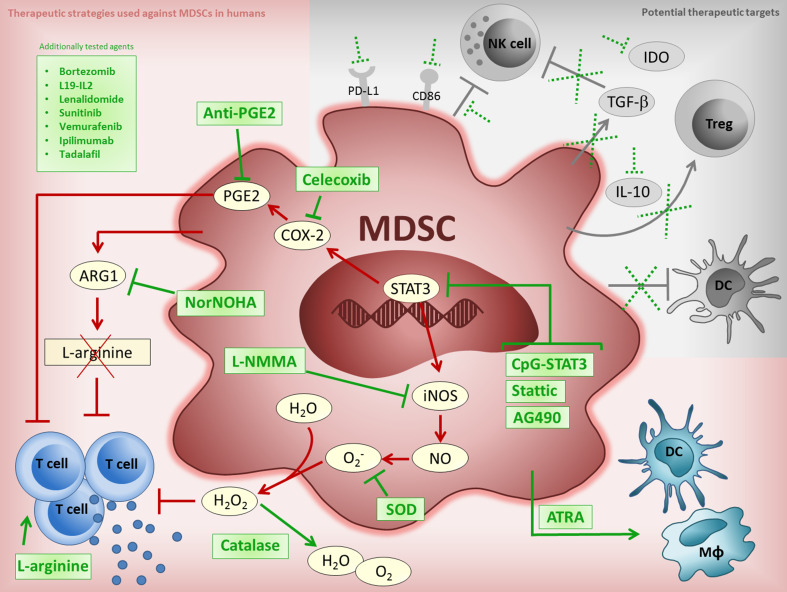
Fig. 1.
Current and potential MDSC targeting strategies. Figure shows the currently tested approaches against human MDSC (red shaded background) and potential therapeutic targets (grey shaded background). Red and grey lines indicate MDSC suppressive pathways, green lines show where therapeutic agents may interfere with these pathways, broken green lines indicate where potential therapeutic agents may act against MDSC suppressive mechanisms, green boxes list previously tested drugs which can interfere with MDSCs. The overlapping suppressive pathway involving STAT3/COX-2/PGE2 can be targeted upstream by STAT3 inhibitors (CpG-STAT3 [53], Stattic [47], AG490 [17]), or further downstream by agents acting against the COX-2 enzyme (Celecoxib) [17] or its product, PGE2 (anti-PGE2 [17]). MDSC depletion of l-arginine via elevated ARG1 levels can result in T cell dysfunction. This may be counteracted by inhibitors of ARG1 (NorNOHA) [17, 53, 109] or by arginine supplementation [97]. ATRA [92, 104, 110] can act to differentiate MDSCs into to non-suppressive cells. Production of reactive oxygen species is a further MDSC mechanism of suppression which can be inhibited upstream via STAT3, by iNOS inhibitors (L-NMMA) [109] or through SOD [17, 110] and catalase [17, 97, 110] directly. Additional agents, such as kinase inhibitors (sunitinib [50, 94, 97, 98], vemurafenib [32]), immunotherapeutics (L19-IL2 [101], ipilimumab [66, 99, 115]) and yet other agents (bortezomib [90, 96, 116], lenalidomide [90, 93, 116], tadalafil [102, 103]) have also been shown to be active against MDSCs. Suppressive mechanisms employed by MDSCs that remain to be exploited for therapy include their effect on DCs, NK cells, Treg induction and via IDO, IL-10, TGF-β, PD-L1 and CD86. ARG1 arginase 1, ATRA all-transretinoic acid, COX-2 cyclooxygenase-2, CpG cytosine-phosphate-guanine oligodeoxynucleotide, DC dendritic cell, IDO indoleamine 2,3-dioxygenase, iNOS inducible nitric oxide synthase, L19-IL2 L19-IL2 monoclonal antibody-cytokine fusion protein, L-NMMA NG-Methyl-l-arginine, MDSC myeloid-derived suppressor cell, MΦ macrophage, NK natural killer cell, Nor-NOHA N(omega)-hydroxy-nor-l-arginine, PD-L1 programmed death-ligand 1, PGE2 prostaglandin E2, SOD superoxide dismutase, STAT3 signal transducer and activator of transcription 3, TGF-β transforming growth factor beta, Treg regulatory T cell
Table 3.
Therapeutic strategies to target MDSCs
| Treatment | MDSC phenotype; sample type | Cancer type | PT | Effect on MDSC level/function/differentiation | Refs. |
|---|---|---|---|---|---|
| Targeting MDSC Level | |||||
| ATRA | CD33+HLA-DR−Lin−; frozen | RCC | ND | Decreased levels | [92] |
| ATRA+DC-p53 | CD33+HLA-DR−Lin−; frozen | SCLC | Yes | Decreased levels | [104] |
| CD14−CD33+CD11b+; frozen | |||||
| DC-p53 | CD33+HLA-DR−Lin−; frozen | SCLC | Yes | No change | [104] |
| CD14−CD33+CD11b+; frozen | |||||
| Bevacicumab | CD66b+; fresh | RCC | ND | No change | [12] |
| Bevacicumab+IL-2 | Increased levels | ||||
| Bortezomib | CD14+HLA-DR−/low; fresh | MM | No | Decreased levels | [96] |
| L19-IL2 treatment | CD14+HLA-DR−/lowCD11b+; frozen | Melanoma | Yes | Decreased levels | [101] |
| Lenalidomide | CD14+HLA-DRlow CD33+CD11b+Lin−; fresh | MM | ND | No change | [93] |
| CD14+CD33+CD15+CD11b+Lin−; fresh | MM | ND | Increased levels | ||
| Lenalidomide or Bortezomib | CD14−HLA-DR−/lowCD11b+; NM | MM | ND | No change | [90] |
| CD15+CD14−HLA-DR−/lowCD33+CD11b+; NM | |||||
| Doxorubicin | CD33+CD11b+HLA-DR−Lin−; fresh | BC | ND | Increased levels | [14] |
| Paclitaxel | No change | ||||
| HSPPC-96+GM-CSF | CD15+; NM | Melanoma | Yes | No change | [8] |
| CD34+; NM | |||||
| HLA-DR−Lin−; NM | |||||
| CD14+CD11b+; NM | Melanoma | Yes | Increased levels | ||
| CD14+HLA-DR−/lowCD11b+; NM | |||||
| Telomerase peptide vaccine (GV1001)+GM-CSF+Gemcitabine+Capecitabine | CD11b+HLA-DR−Lin−; frozen | PC | ND | Decreased levels | [95] |
| Gemcitabine+Capecitabine | CD11b+HLA-DR−Lin−; frozen | PC | ND | No consistent reduction | [95] |
| CDDO-Me+Gemcitabine | CD33+HLA-DR−Lin−; NM | PAC | ND | No change | [105] |
| CD14−CD33+CD11bhi; NM | |||||
| Sunitinib | CD15+CD14−; frozen | RCC | Yes | Decreased levels | [97] |
| HLA-DR−CD33+; frozen | |||||
| CD15+HLA-DR−CD33+CD11c+; frozen | |||||
| CD33+HLA-DR−CD15−CD11c+; frozen | |||||
| CD14+HLA-DR−/low; NM | RCC | Yes | Decreased levels | [94] | |
| CD11c+HLA-DR−Lin−; NM | RCC | Yes | Increased levels | ||
| CD33+CD11b+HLA-DR−; IF tissue | RCC | ND | Undetectable levels | [98] | |
| CD33+HLA-DR−CD14−CD15−; tumour digest | Decreased levels | ||||
| CD15+HLA-DR−CD33+; tumour digest | |||||
| CD14+HLA-DR−CD33+; tumour digest | No change | ||||
| CD14+CD33+CD16+; frozen | Mix | ND | Decreased levels | [50] | |
| CD14+CD33+CD16−; frozen | |||||
| Vemurafenib | CD14+HLA-DR−/lowCD15dimCD33+CD11b+CD16−/lowCD45+CD66b−Arginase1−Lin−; NM | Melanoma | No | PR: decrease | [32] |
| CD15+CD14−HLA-DR−/lowCD33+CD11b+CD16−/lowCD45+CD66b+CD11b+Arginase1+Lin−; NM | |||||
| Targeting MDSC Level | |||||
| Ipilimumab | CD15+HLA-DR−/lowCD33+CD11b+Lin−; fresh | Melanoma | Yes | Decreased levels | [99] |
| CD14+HLA-DR−/lowCD3−CD19−; fresh | Melanoma | Yes | No change | ||
| CD15+HLA-DR−/lowCD11b+SSClow; frozen | Melanoma | No | No change | [100] | |
| CD14+HLA-DR−/lowCD11b+SSClow; frozen | Melanoma | No | Decreased or Increased levels | ||
| CD14+HLA-DR−Lin−; fresh | Melanoma | Yes | No change | [66] | |
| Tadalafil | CD14+HLA-DRint/lowCD33+CD11b+IL-4Ra+; frozen | HNSCC | Yes | Decreased levels | [102] |
| CD33+IL-4Ra+; Tissue | Decreased levels (trend) | ||||
| Not clearly defined | HNSCC | Yes | Decreased levels | [103] | |
| CpG-STAT3 siRNA | CD15+CD14−; fresh | Prostate | Yes | No change | [53] |
| Zoledronic acid | CD15+CD33+CD11b+CD45+Lin−; frozen | PAC | No | No change | [107] |
| VEGF-trap | CD33+HLA-DR−Lin−; frozen | mix | ND | No change | [106] |
| Targeting MDSC function | |||||
| L-arginine | CD15+CD33+; fresh | RCC | Yes | Restored T cell function | [97] |
| Catalase | CD15+CD33+; fresh | RCC | Yes | Restored T cell function | [97] |
| CD33+HLA-DR−; fresh | RCC | ND | Restored T cell function | [110] | |
|
CD14+; fresh CD14+; fresh |
In vitro Melanoma |
No ND |
Weakly restored T cell function Restored T cell function |
[17] | |
| ATRA | CD33+HLA-DR−; fresh | RCC | ND | Restored T cell function | [110] |
| Anti-PD-1/PD-L1+Lenalidomide | CD14+HLA-DR−/lowCD11b+; NM | MM | ND |
Restored T/NK/NKT cell function Reduced tumour cell growth |
[116] |
| Lenalidomide or Bortezomib | CD15+CD14−HLA-DR−/lowCD33+CD11b+; NM | MM | ND |
No restoration in T cell function Decreased levels of COX-2, IL-6, IL-10, IFNγ and GM-CSF in MDSC |
[116] |
| CDDO-Me+Gemcitabine | CD33+Lin−HLA-DR−; NM | RCC | ND | Restored T cell function | [105] |
| CD14−CD33+CD11bhi; NM | |||||
| Sunitinib | CD15+CD33+; fresh | RCC | Yes | Restored T cell function | [97] |
| CD14+CD33+CD16+; fresh | Mix | ND |
In vitro: Restored T cell function and decreased pSTAT3, CD206, ARG; increased iNOS; in vivo: Decreased ARG and pSTAT3 |
[50] | |
| Ipilimumab | CD3− fresh | Melanoma | Yes | Decreased frequency of ARG+cells | [99] |
| CD15+HLA-DR−/lowCD33+CD11b+Lin−; fresh | |||||
| CD15+HLA-DR−/lowCD11b+SSClow; frozen | Melanoma | No | No change in NO | [100] | |
| CD14+HLA-DR−/lowCD11b+SSClow; frozen | Melanoma | No | No change in PD-1 | ||
| Tadalafil | CD15+; NM | HNSCC | Yes | Decreased iNOS and ARG | [103] |
| Targeting MDSC function | |||||
| CpG-STAT3 siRNA | CD15+CD14−; fresh | Prostate | Yes |
Restored T cell function; Decreased ARG |
[53] |
| AG490 (STAT3 inhibitor) | CD14+; fresh | In vitro | No | Restored T cell function (trend) | [17] |
| CD14+; fresh | Melanoma | ND | Restored T cell function | ||
| Stattic and siRNA inhibition of STAT3 | CD14+HLA-DR−/low; fresh | HNSCC | ND |
Restored T cell function; Decrease ARG |
[47] |
| Celecoxib (COX-2 inhibitor) | CD14+; fresh | In vitro | No | Restored T cell function | [17] |
| CD14+; fresh | Melanoma | ND | Restored T cell function (trend) | ||
| Neutralizing TGF-β |
CD14+; fresh CD14+; fresh |
In vitro Melanoma |
No ND |
Restored T cell function (weak/inconsistent) Restored T cell function (trend) |
[17] |
| Anti-PGE2 | CD14+; fresh | Melanoma | ND | Restored T cell function | [17] |
| Superoxide dismutase | CD14+; fresh | In vitro | No | Restored T cell function (weak/inconsistent) | [17] |
| Superoxide dismutase | CD33+HLA-DR−; fresh | RCC | ND | No change | [110] |
| Amiloride (decreases exosome production) | CD33+HLA-DR−CD3−; NM | CRC | ND | Decreased pSTAT3 | [111] |
| Depleted phenotype not given; NM | Restored T cell function | ||||
| VEGF-Trap | CD33+HLA-DR−Lin−; frozen | Mix | ND |
Restored T cell function (if MDSC levels decreased) No restoration of T cell function (if MDSC levels increased) |
[106] |
| Sildenafil | CD14+; fresh | MM | ND | Restored T cell function | [109] |
| Nor-NOHA (ARG inhibitor)+L-NMMA (NOS inhibitor) | |||||
| Nor-NOHA (ARG inhibitor) | CD15+CD14−; fresh | Prostate | Yes | Restored T cell function | [53] |
| CD14+; fresh | In vitro | No | Restored T cell function (weak/inconsistent) | ||
| CD14+; fresh | Melanoma | ND | Restored T cell function (trend) | [17] | |
| Targeting MDSC differentiation | |||||
| ATRA | CD33+HLA-DR−; fresh | RCC | ND | Increase in CD1a, HLA-DR and CD40 expression | [110] |
| HSPPC-96+GM-CSF | CD14+HLA-DR−/lowCD11b+; NM | Melanoma | Yes | No change in co-stimulatory molecules | [8] |
| Sunitinib | CD33+HLA-DR−; fresh | RCC | Yes | No change in MHC II/co-stimulatory molecules; no effect on induction of MDSC differentiation | [97] |
| Celecoxib (COX-2 inhibitor) or AG490 (STAT3 inhibitor) | CD14+; fresh | In vitro | No | Induction inhibition of MDSC-like phenotype | [17] |
| Vemurafenib | CD14+HLA-DR−/low; NM | In vitro | No | No effect on induction of MDSC-like phenotype | [32] |
ARG arginase, ATRA all-transretinoic acid, BC breast cancer, CDDO-Me bardoxolone methyl,COX-2 cyclooxygenase-2, CpG cytosine-phosphate-guanine oligodeoxynucleotide, CRC colorectal cancer, DC dendritic cell, HNSCC head and neck squamous cell carcinoma, HSPPC-96 Heat shock protein peptide complex-96, iNOS inducible nitric oxide synthase, MM multiple myeloma, ND no data, NM not mentioned, NO nitric oxide, PAC pancreatic adenocarcinoma, PC pancreatic cancer, PD-1 Programmed cell death, PD-L1 programmed death-ligand 1, PGE2 prostaglandin E2, PR Partial Responders, PT pretreatment, RCC renal cell carcinoma, ROS reactive oxygen species, SCLC small cell lung cancer, STAT3 signal transducer and activator of transcription 3, VEGF Vascular endothelial growth factor
The most common therapeutic approach against MDSCs so far has been aimed at reducing their level. Of the 30 studies which report therapeutic intervention against MDSCs in humans, we identified 25 representing at least 23 different therapies aimed at altering the level of MDSCs in a range of human cancers. It is interesting that differences have been shown for any given therapy—some have reported an increase [8, 12, 14, 92–94], decrease [32, 50, 92, 94–104], or no change [8, 12, 34, 53, 90, 93, 95, 99, 100, 104–107] in the level of MDSCs, even in a particular cancer type with the same therapy. For example, Van Cruijsen et al. [94] reported an increase in the level of immature myeloid cells upon treatment with sunitinib, whereas Ko et al. [97] and Guislain et al. [98] observed a decrease in a different MDSC phenotype. This could be due to the possible pleiotropic effects of sunitinib, because it is a multikinase inhibitor of vascular endothelial growth factor receptors 1-3 (VEGFR1-3), platelet-derived growth factor receptor (PDGFR) a and b, stem cell factor (c-Kit), FMS-like tyrosine kinase 3 (FLT3) and macrophage colony-stimulating factor (M-CSF) receptor, which are all involved in the recruitment, expansion and activation of MDSCs and which have receptors on both tumour cells and MDSCs. Moreover, a recent publication suggests that sunitinib may be a poor inhibitor of MDSC induction compared to certain other tyrosine kinase blockers, such as nilotinib, dasatinib and sorafenib [108]. Comparing the results of different reports in this manner highlights a major difficulty in the study of MDSCs in general because different studies have used different phenotypes to characterise MDSCs, making direct comparisons difficult. Due to these differences in phenotypes across studies, this also makes it challenging to pair together studies which have shown an effective targeting strategy against particular MDSC phenotypes with other studies which report that the level of certain MDSC phenotypes in the same cancer type are clinically relevant to patients with this cancer. Another example of different effects reported for the same therapy is ipilimumab in melanoma patients. Pico de Coana et al. [99] reported a decrease in the level of grMDSCs, whereas Gebhardt et al. [100] observed no change. Given that granulocytes and grMDSC are sensitive to freezing [51, 79], this might reflect an experimental artefact rather than represent a biological difference because the former study used fresh PBMCs while frozen samples were used by Gebhardt et al. [100], or alternately this may be due to differences in the pretreatment of the patients.
As highlighted in the previous section, current studies have identified a small number of mechanisms used by MDSCs to exert their suppressive function. These common mechanisms include Treg induction, ROS, arginase, TGF-β production and the overlapping PGE2/COX-2/STAT3 pathways which, therefore, represent potential targets for therapeutic intervention against MDSCs. We identified 15 studies which reported on drugs that target many of these suppressive mechanisms in at least five different entities of human cancer. All of these studies showed that these drugs modulate the pathways involved in arginase [50, 53, 80, 99, 103, 109], ROS [97, 110], COX-2 [80, 110], STAT3 [47, 50, 53, 80, 111] and to restore T cell proliferation and/or function [47, 50, 53, 80, 97, 103, 105, 106, 109–111]. Of note is that many studies did not test if abrogation of MDSC-mediated suppression was due to inhibition of the suppressive pathway or due to other possible mechanisms, such as apoptosis induction. For example, using T cell/MDSC co-culture suppression assays, Nagaraj et al. [105] observed an increase in IFN-γ production in patients with RCC upon treatment with the IκB inhibitor CDDO-Me, but the authors did not investigate by which mechanism the improved IFN-γ production occurred. Also using co-culture suppression assays, Ko et al. [97], demonstrated that sunitinib resulted in improved T cell function and that this occurred through the induction of apoptosis in MDSCs. This highlights the importance of distinguishing between the possible different therapeutic mechanisms of these drugs.
Strategies manipulating the differentiation of MDSCs can be achieved by either blocking the differentiation of myeloid cells into MDSCs or by driving the differentiation of MDSCs into non-suppressive cells. To date, this remains the least studied approach with only a handful of investigations attempting this strategy. We were able to identify five studies using this approach, four of which were performed in vitro [32, 80, 97, 110], with the remaining study performed in cancer patients in vivo [8]. One of these studies failed to show changes with vemurafenib on the differentiation of MDSCs in vitro [32], while two showed no change in expression of MHC II or co-stimulatory molecules on MDSCs from patients with metastatic melanoma or RCC treated with Heat shock protein peptide complex-96 (HSPPC-96) in combination with GM-CSF vaccination [8] or sunitinib [97], respectively. In contrast, Kusmartsev et al. [110] demonstrated that ATRA in combination with GM-CSF induced the differentiation of suppressive myeloid cells to those with a more mature phenotype in vitro. Another mechanism of targeting the differentiation of MDSCs was reported by Mao et al. [80] in in vitro co-culture studies where blocking COX-2 or STAT3 resulted in the inhibition of tumour-induced MDSC-mediated T cell suppression, with similar effects reported when these pathways were directly inhibited on patient derived MDSCs. Of the current targeting strategies, manipulation of MDSC differentiation is the least studied approach in humans.
High MDSC levels are associated with worse clinical outcome in many cancer types, but it has not yet been shown whether MDSC targeted therapies result in patient benefit. Therefore, future studies should be more focused on identifying the clinical effects of such agents. The range of therapies investigated as MDSC targeting drugs so far represent a diverse group of agents that vary considerably in target specificity. It is therefore not precisely clear how these agents function in their interaction with MDSCs, and one factor not taken into consideration when analysing the effect of a given therapy on the level of MDSCs is the effect of the therapy on the tumour which may indirectly result in changes to MDSCs. An association between disease stage and MDSC level has been reported in numerous cancer types (see “MDSC levels in human cancer and response to therapy”). Thus, a therapy-induced reduction of peripheral MDSCs may reflect activity against the tumour and may not be directly related to an effect on MDSCs. To determine the mechanism by which any observed therapeutic effect occurs, future studies will need to address this issue. For example, the cell types responsive to the employed therapeutic regimen may be assessed against individual cell types in vitro. Despite the exact mode of action remaining unclear, the combination of MDSC therapies may be one method of effectively combating MDSCs. For example, the combination of agents that act to reduce MDSCs (such as Gemcitabine [95]) with those that promote their differentiation (e.g., ATRA [92, 110]) may be a useful approach that could result in more efficacious MDSC directed therapies. Noteworthy is that none of these therapies were originally developed for the purpose of MDSC targeting, as illustrated by ipilimumab which was designed to act on T cells but also happens to have an effect on MDSCs. These results suggest that approaches which specifically target MDSCs may be more successful in the future.
Discussion
In this review, we have surveyed studies which collectively unequivocally document that MDSCs are important players in human cancer. However, it remains to be seen if any of the reported strategies which target MDSCs in cancer patients in vivo result in improvement in patient status. We propose the following three step procedure for the clinical implementation of MDSC-based therapeutics. Step 1: Although we understand MDSCs to be of general importance to cancer, validated and reproduced clinical associations from multiple laboratories exist for only a few cancer types. This is particularly the case for the most important clinical feature; patient survival. We therefore suggest that future efforts should be more heavily focused on precisely defining and validating clinical associations for each cancer type. Only then can it be investigated if certain cancer patient groups may benefit from MDSC targeted therapies. Step 2: To facilitate the testing and development of MDSC targeted therapies, a better understanding of how MDSCs mediate immune suppression will be required. To allow the development of a range of therapies, a full spectrum of potential target mechanisms and an understanding of how each of these suppress the immune system will be required. Step 3: In the cases where robust clinical relationships have been defined, MDSC targeted therapies should then be tested. This could be performed initially in vitro using patient material, but subsequently in vivo in animals (primarily to estimate potential toxicity) and humans (to directly test the clinical effect of such drugs). In this context, it is not known if drugs which target MDSCs in general will be effective (in which case less emphasis can be placed on Step 2), or if drugs which target particular suppressive mechanisms in each cancer type or in each patient will be required to deliver clinical benefit. As such, Steps 1 and 2 will allow an understanding of the therapeutic mechanism of each drug and the suppressive mechanism of different MDSC. Pairing these may ultimately allow the matching of therapies which target particular suppressive mechanism(s) to these patients. This would most likely benefit patients who have high levels of MDSCs utilising a particular suppressive mechanism in a cancer type in which the negative role of MDSCs has been established.
Despite the proliferation of MDSC-focused investigation, there is a relative dearth of literature on their role in humans. Our inspiration behind this work was to more clearly define the state of the art of MDSCs exclusively in the setting of human cancer. This allowed us to identify where progress has been made and also where the problems are in the field as a whole. We found a number of studies which showed that MDSCs are clinically relevant in different cancer types. It is not yet clear if this is because MDSCs are important in all cancer types or only in some, or if the lack of negative reports is due to publication bias for the preferential reporting of significant results [112–114]. One of the common themes running across the different aspects of this review is the lack of standard protocols for assessing MDSCs. This contributes to the difficulty of comparing results from different studies. There have been recent attempts [78] to harmonise MDSC phenotype assessment but many other aspects also require attention, especially in the case of standardising conditions for the functional testing of MDSCs. Even so, employing standards will not entirely overcome some of the most pressing questions. Primarily due to technical limitations and differences in investigator preference, it is still an open question if the similar or different phenotypes reported across studies represent distinct or overlapping cellular populations. Addressing these widespread issues while following our proposed three step procedure may lead to the more rapid translation of MDSC targeted therapies which may improve the treatment of cancer.
Acknowledgments
This work was supported by Grants from the German Research Foundation (GP) (DFG Pa 361/22-1) and the fortüne program of the University Hospital Tübingen (CS) (F 1282980).
Contributor Information
Christopher Shipp, Email: christopher.shipp@uni-tuebingen.de, Email: mrchristophershipp@gmail.com.
Graham Pawelec, Email: graham.pawelec@uni-tuebingen.de.
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A. The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol. 2010;40:3317–3320. doi: 10.1002/eji.201041170. [DOI] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdani Y, Mohammadnia-Afrouzi M, Yousefi M, Anvari E, Ghalamfarsa G, Hasannia H, et al. Myeloid-derived suppressor cells in B cell malignancies. Tumour Biol. 2015;36:7339–7353. doi: 10.1007/s13277-015-4004-z. [DOI] [PubMed] [Google Scholar]
- 6.Motallebnezhad M, Jadidi-Niaragh F, Qamsari ES, Bagheri S, Gharibi T, Yousefi M (2015) The immunobiology of myeloid-derived suppressor cells in cancer. Tumour Biol 37:1387–1406 [DOI] [PubMed]
- 7.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144:250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 9.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 10.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 11.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–922. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20:4096–4106. doi: 10.1158/1078-0432.CCR-14-0635. [DOI] [PubMed] [Google Scholar]
- 17.Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, et al. Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 2014;193:2574–2586. doi: 10.4049/jimmunol.1400833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 20.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, et al. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, et al. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med. 2011;9:90. doi: 10.1186/1479-5876-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 26.Wesolowski R, Markowitz J, Carson WE., 3rd Myeloid derived suppressor cells—a new therapeutic target in the treatment of cancer. J Immunother Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudolph BM, Loquai C, Gerwe A, Bacher N, Steinbrink K, Grabbe S, et al. Increased frequencies of CD11b(+) CD33(+) CD14(+) HLA-DR(low) myeloid-derived suppressor cells are an early event in melanoma patients. Exp Dermatol. 2014;23:202–204. doi: 10.1111/exd.12336. [DOI] [PubMed] [Google Scholar]
- 28.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, et al. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother. 2013;62:1711–1722. doi: 10.1007/s00262-013-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, et al. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. 2015;136:2352–2360. doi: 10.1002/ijc.29297. [DOI] [PubMed] [Google Scholar]
- 30.Gros A, Turcotte S, Ahmadzadeh M, Wunderlich JR, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T cell proliferation in patients with melanoma. Cancer Immunol Immunother. 2012;18:5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitano S, Postow MA, Ziegler CG, Kuk D, Panageas KS, Cortez C, et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res. 2014;2:812–821. doi: 10.1158/2326-6066.CIR-14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Gorgens A, et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133:1653–1663. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 33.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+ HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 34.Meyer H, Cagnon G, Costa-Nunes G, Baumgaertner H, Montandon X, Leyvrazn X, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2013;63:247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idorn M, Kollgaard T, Kongsted P, Sengelov L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63:1177–1187. doi: 10.1007/s00262-014-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan XK, Zhao XK, Xia YC, Zhu X, Xiao P. Increased circulating immunosuppressive CD14(+)HLA-DR(-/low) cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J Int Med Res. 2011;39:1381–1391. doi: 10.1177/147323001103900424. [DOI] [PubMed] [Google Scholar]
- 38.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, et al. Increase in CD14+HLA-DR−/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62:1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vetsika EK, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, et al. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res. 2014;2014:659294. doi: 10.1155/2014/659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Zhou Y, Huang Q, Qiu L. CD14HLA-DR expression: a novel prognostic factor in chronic lymphocytic leukemia. Oncol Lett. 2015;9:1167–1172. doi: 10.3892/ol.2014.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, et al. IL-6-stimulated CD11b+CD14+HLA-DR− myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5:8716–8728. doi: 10.18632/oncotarget.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, Zhang G, Li G, Ma H, Zhang X. Circulating CD14HLA-DR myeloid-derived suppressor cell is an indicator of poor prognosis in patients with ESCC. Tumour Biol. 2015;36:7987–7996. doi: 10.1007/s13277-015-3426-y. [DOI] [PubMed] [Google Scholar]
- 45.Romano A, Parrinello NL, Vetro C, Forte S, Chiarenza A, Figuera A, et al. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin lymphoma patients treated up-front with a risk-adapted strategy. Br J Haematol. 2015;168:689–700. doi: 10.1111/bjh.13198. [DOI] [PubMed] [Google Scholar]
- 46.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 47.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H, Zhang G, Li G, Ma H, Zhang X. Circulating CD14(+)HLA-DR(-/low) myeloid-derived suppressor cell is an indicator of poor prognosis in patients with ESCC. Tumour Biol. 2015;36:7987–7996. doi: 10.1007/s13277-015-3426-y. [DOI] [PubMed] [Google Scholar]
- 49.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(-)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 50.Chen HM, Ma G, Gildener-Leapman N, Eisenstein S, Coakley BA, Ozao J, et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin Cancer Res. 2015;21:4073–4085. doi: 10.1158/1078-0432.CCR-14-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trellakis S, Bruderek K, Hutte J, Elian M, Hoffmann TK, Lang S, et al. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun. 2013;19:328–336. doi: 10.1177/1753425912463618. [DOI] [PubMed] [Google Scholar]
- 52.Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, et al. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–1119. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]
- 53.Hossain DM, Pal SK, Moreira D, Duttagupta P, Zhang Q, Won H, et al. TLR9-targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin Cancer Res. 2015;21:3771–3782. doi: 10.1158/1078-0432.CCR-14-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khaled YS, Ammori BJ, Elkord E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J Immunol Res. 2014;2014:879897. doi: 10.1155/2014/879897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 56.Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, et al. CD15+/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol. 2012;33:121–129. doi: 10.1007/s13277-011-0254-6. [DOI] [PubMed] [Google Scholar]
- 57.Wu WC, Sun HW, Chen HT, Liang J, Yu XJ, Wu C, et al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci USA. 2014;111:4221–4226. doi: 10.1073/pnas.1320753111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chi N, Tan Z, Ma K, Bao L, Yun Z. Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and -6 in prostate cancer. Int J Clin Exp Med. 2014;7:3181–3192. [PMC free article] [PubMed] [Google Scholar]
- 59.Chevolet I, Speeckaert R, Schreuer M, Neyns B, Krysko O, Bachert C, et al. Clinical significance of plasmacytoid dendritic cells and myeloid-derived suppressor cells in melanoma. J Transl Med. 2015;13:9. doi: 10.1186/s12967-014-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 61.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun HL, Zhou X, Xue YF, Wang K, Shen YF, Mao JJ, et al. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J Gastroenterol. 2012;18:3303–3309. doi: 10.3748/wjg.v18.i25.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64:149–159. doi: 10.1007/s00262-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mabuchi S, Matsumoto Y, Kawano M, Minami K, Seo Y, Sasano T, et al (2014) Uterine cervical cancer displaying tumor-related leukocytosis: a distinct clinical entity with radioresistant feature. J Natl Cancer Inst 106(7):dju147. doi:10.1093/jnci/dju147 [DOI] [PubMed]
- 65.Wang D, An G, Xie S, Yao Y, Feng G (2016) The clinical and prognostic significance of CD14HLA-DR myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. (Epub ahead of print) [DOI] [PubMed]
- 66.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63:247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2012;61:827–838. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20:1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 70.Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol. 2012;30:1835–1841. doi: 10.1200/JCO.2011.40.2271. [DOI] [PubMed] [Google Scholar]
- 71.Bailur JK, Gueckel B, Derhovanessian E, Pawelec G. Presence of circulating Her2-reactive CD8+ T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. 2015;17:34. doi: 10.1186/s13058-015-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 73.Filipazzi P, Pilla L, Mariani L, Patuzzo R, Castelli C, Camisaschi C, et al. Limited Induction of Tumor-cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen-class I-modified peptides. Clin Cancer Res. 2012;18:6485–6496. doi: 10.1158/1078-0432.CCR-12-1516. [DOI] [PubMed] [Google Scholar]
- 74.Martens A, Wistuba-Hamprecht K, Geukes Foppen MH, Yuan J, Postow MA, Wong P, et al (2016) Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 75.Santegoets SJ, Stam AG, Lougheed SM, Gall H, Jooss K, Sacks N, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer. 2014;2:31. doi: 10.1186/s40425-014-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansen GL, Gaudernack G, Brunsvig PF, Cvancarova M, Kyte J. Immunological factors influencing clinical outcome in lung cancer patients after telomerase peptide vaccination. Cancer Immunol Immunother. 2015;64:1609–1621. doi: 10.1007/s00262-015-1766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One. 2014;9:e87705. doi: 10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65:161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Brage SE, Schultz I, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93:273–278. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez PC, Quiceno DG, Ochoa AC. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshimura A, Muto G. TGF-beta function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–147. doi: 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 86.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kortylewski M, Yu H. Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol. 2008;20:228–233. doi: 10.1016/j.coi.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124:750–760. doi: 10.1182/blood-2013-12-546416. [DOI] [PubMed] [Google Scholar]
- 89.Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A (2014) Inhibition of tumor-derived prostaglandin-E2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res [DOI] [PubMed]
- 90.Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121:2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chikamatsu K, Sakakura K, Toyoda M, Takahashi K, Yamamoto T, Masuyama K. Immunosuppressive activity of CD14+HLA-DR−cells in squamous cell carcinoma of the head and neck. Cancer Sci. 2012;103:976–983. doi: 10.1111/j.1349-7006.2012.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Busch A, Zeh D, Janzen V, Mugge LO, Wolf D, Fingerhut L, et al. Treatment with lenalidomide induces immunoactivating and counter-regulatory immunosuppressive changes in myeloma patients. Clin Exp Immunol. 2014;177:439–453. doi: 10.1111/cei.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Cruijsen H, van der Veldt AA, Vroling L, Oosterhoff D, Broxterman HJ, Scheper RJ, et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14:5884–5892. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 95.Annels NE, Shaw VE, Gabitass RF, Billingham L, Corrie P, Eatock M, et al. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63:175–183. doi: 10.1007/s00262-013-1502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Z, Zhang L, Wang H, Xiong S, Li Y, Tao Q, et al. Tumor-induced CD14+HLA-DR (-/low) myeloid-derived suppressor cells correlate with tumor progression and outcome of therapy in multiple myeloma patients. Cancer Immunol Immunother. 2015;64:389–399. doi: 10.1007/s00262-014-1646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 98.Guislain A, Gadiot J, Kaiser A, Jordanova ES, Broeks A, Sanders J, et al. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol Immunother. 2015;64:1241–1250. doi: 10.1007/s00262-015-1735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pico de Coana Y, Poschke I, Gentilcore G, Mao Y, Nystrom M, Hansson J, et al. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res. 2013;1:158–162. doi: 10.1158/2326-6066.CIR-13-0016. [DOI] [PubMed] [Google Scholar]
- 100.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al (2015) Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin Cancer Res [DOI] [PubMed]
- 101.Weide B, Eigentler TK, Pflugfelder A, Zelba H, Martens A, Pawelec G, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2:668–678. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 102.Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–38. doi: 10.1158/1078-0432.CCR-14-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 107.Sanford DE, Porembka MR, Panni RZ, Mitchem JB, Belt BA, Plambeck-Suess SM, et al. A study of Zoledronic acid as neo-adjuvant, perioperative therapy in patients with resectable pancreatic ductal adenocarcinoma. J Cancer Ther. 2013;4:797–803. doi: 10.4236/jct.2013.43096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heine A, Schilling J, Grunwald B, Kruger A, Gevensleben H, Held SA, et al (2016) The induction of human myeloid derived suppressor cells through hepatic stellate cells is dose-dependently inhibited by the tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but not sunitinib. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed]
- 109.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 111.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14:iii, ix–xi, 1–193 [DOI] [PubMed]
- 113.McGauran N, Wieseler B, Kreis J, Schuler YB, Kolsch H, Kaiser T. Reporting bias in medical research—a narrative review. Trials. 2010;11:37. doi: 10.1186/1745-6215-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 115.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. 2015;21:5453–5459. doi: 10.1158/1078-0432.CCR-15-0676. [DOI] [PubMed] [Google Scholar]
- 116.Gorgun G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. 2015;21:4607–4618. doi: 10.1158/1078-0432.CCR-15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–6541. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 118.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2011;61:827–838. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]