Fig. 2.
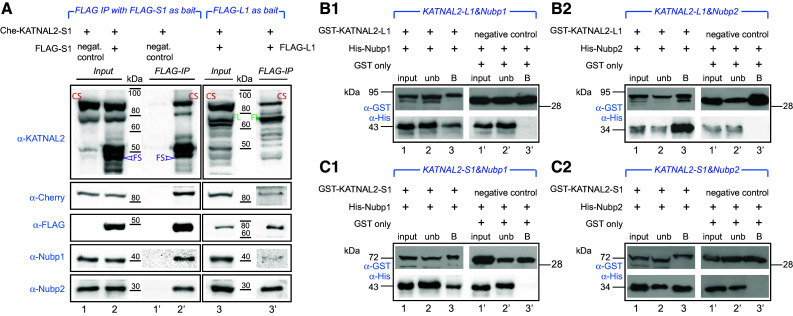
Nubp1 and Nubp2 interact with KATNAL2 in vivo and in vitro, and KATNAL2s interact with themselves. a The Cherry-KATNAL2-S1 (“Che-KATNAL2-S1”) stable cell line was transiently transfected with plasmids expressing as bait either FLAG-KATNAL2-S1 (“FLAG-S1”) or FLAG-KATNAL2-L1 (“FLAG-L1”) (as indicated at the header for each set) or mock empty vector plasmid (negative control) and subjected to IP with anti-FLAG beads. The presence of Cherry-KATNAL2-S1, KATNAL2s, FLAG, Nubp1 and Nubp2 was probed by WB with appropriate antibodies as shown. Results show that the FLAG-KATNAL2-S1 or -L1 bait co-immunoprecipitates Nubp1 and Nubp2 but also the “endogenous” Cherry-KATNAL2-S1 and several native KATNAL2 (different bands migrating in positions within ca. 40–80 kDa). All these proteins are absent from the negative control IP reaction (lane 1′). CS, Cherry-KATNAL2-S1; FS, FLAG-KATNAL2-S1; FL, FLAG-KATNAL2-L1). b1–c2 Recombinant GST-KATNAL2-L1 or -S1 and His-Nubp1 or His-Nubp2 were probed for pairwise interactions (as indicated for each set in the blue header). GST-only was used in conjunction with His-Nubp1 or His-Nubp2 in parallel negative control reactions. Complexes were immobilized on glutathione beads, eluted, and two equal parts were analyzed by WB, either using anti-GST (to detect the KATNALs) or anti-His (to detect the Nubps). Results show that Nubp1 or Nubp2 is detectable on the glutathione beads only in the presence of a KATNAL2 and indicate that both KATNAL2-L1 and KATNAL2-S1 interact individually with both Nubp1 and Nubp2. Input corresponds to 1/20 volume of the lysate used for the reaction, unb is the unbound fraction after incubation of the mixed recombinant protein lysates with glutathione beads, B is the bound fraction corresponding to preformed protein complexes, captured on the beads
