Abstract
During starch metabolism, the phosphorylation of glucosyl residues of starch, to be more precise of amylopectin, is a repeatedly observed process. This phosphorylation is mediated by dikinases, the glucan, water dikinase (GWD) and the phosphoglucan, water dikinase (PWD). The starch-related dikinases utilize ATP as dual phosphate donor transferring the terminal γ-phosphate group to water and the β-phosphate group selectively to either C6 position or C3 position of a glucosyl residue within amylopectin. By the collaborative action of both enzymes, the initiation of a transition of α-glucans from highly ordered, water-insoluble state to a less order state is realized and thus the initial process of starch degradation. Consequently, mutants lacking either GWD or PWD reveal a starch excess phenotype as well as growth retardation. In this review, we focus on the increased knowledge collected over the last years related to enzymatic properties, the precise definition of the substrates, the physiological implications, and discuss ongoing questions.
Keywords: Starch metabolism; Glucan, water dikinase; Phosphoglucan, water dikinase; Starch phosphorylation; Starch degradation
Introduction
Starch is an α-d-glucan that consists of two polymers, amylose and amylopectin. In both polymers, glucosyl residues are predominantly linked by α-1,4-glycosidic bonds and with a lesser frequency by α-1,6-glycosidic bonds. The former interglucose-linkage leads to linear glucan chains, while the latter introduces branch points. Amylose is defined as an essentially linear molecule containing no or very few α-1,6-glycosidic bonds.
In contrast, in the amylopectin molecule the proportion of α-1,6-linkages is higher, which accounts for 5–6 % of total linkages [1]. The branch points are clustered rather than randomly distributed over the molecule. Thereby, neighboring linear glucan chains form double helices. On the next higher structural level, these double helices arrange in an ordered crystalline way and, according to the mode of ordering, at least two different starch crystalline allomorphs can be distinguished. In the A-type allomorph, the double helices form a monoclinic unit cell [2], whereas in the B-type allomorph the double helices organize in a hexagonal unit cell [3]. Irrespective of the allomorph, the double helices form crystalline lamellae. However, in the amorphous lamellae, the branch points are located. This semi-crystalline structure of native starch granules is not observed for other glucan storage polymers, such as glycogen. The water-soluble glycogen is the storage carbohydrate of bacteria and certain eukaryotes, such as fungi and animals. Like amylopectin, glycogen is a polymer that exclusively consists of glucosyl residues that are interconnected by α-1,4- and α-1,6-linkages. Unlike amylopectin, the formation of crystalline lamellae is impeded as α-1,6-glycosidic bonds are not clustered. According to the current models, starch metabolism evolved from an ancient glycogen metabolism and, therefore, contains a setup of enzyme activities of prokaryotic, as well as eukaryotic origin [4, 5]. Nevertheless, the evolution of semi-crystalline starch granules was accompanied by the evolution of enzymes that significantly act on semi-crystalline surfaces and are not found elsewhere [5]. Most probably, these enzymatic activities arose during the transition of a soluble to a water-insoluble storage glucan.
In the chlorophyta lineage (green algae and land plants), starch is exclusively localized in the plastid. In higher plants, starch is found in chloroplasts of photoautotrophic source tissues/organs, such as leaves, and in amyloplasts of heterotrophic tissues/organs, such as seeds and tubers, depending on its function as a diurnal carbon storage or a long-time reserve, respectively. In autotrophic leaf cells, the so-called transitory starch is synthesized in the light phase driven by photosynthetic carbon dioxide fixation. In a diurnal rhythm, transitory starch is degraded during subsequent dark phases, ensuring a constant carbon and energy supply for cellular metabolism at night [6]. In heterotrophic cells of seeds and tubers, reserve starch is formed over a period of days and weeks [7, 8]. Additionally, the synthesis of starch and other reserves in these organs strictly relies on the carbon supply from the mother plant. After a period of dormancy, starch is mobilized in seeds and tubers during germination [9] and late stages of sprouting [10, 11], respectively. Thus, the starch storing tissue/organ undergoes major physiological changes during the process of reserve starch formation and mobilization. In this respect, the degradation of reserve starch is not strictly dependent on cellular integrity. For example, reserve starch stored in endosperm cells of cereal seeds is degraded following seed maturation and programmed cell death of the endosperm [12]. Thus, leaf starch metabolism is more suitable for the analysis of repetitively occurring starch turnover (the process of synthesis and degradation).
Transitory starch turnover in Arabidopsis
On basis of genomic, transcriptomic, proteomic, and metabolomic approaches, Arabidopsis thaliana is the most often analyzed model plant, which is why we will focus on results gained from this model system in this section.
Using forward and reverse genetics, up to now, more than 40 enzymes were discovered that are active in the process of transitory starch turnover in Arabidopsis. The majority of starch-related enzymes are functionally located inside the plastid and have, therefore, direct access to the native starch granules. Starch synthases (SS), branching enzymes (BE) and debranching enzymes (DBE) catalyze the formation of the granules (for more information, see Pfister and Zeeman [13]). Amylolytic and phosphorolytic enzymes, such as amylases and phosphorylases, catalyze the hydrolysis or phosphorolysis of the starch polymer, respectively.
The Arabidopsis genome encodes for several amylase-like proteins that were shown to be located in the chloroplast [14–18]. α-Amylases (AtAMY) and β-amylases (AtBAM) hydrolyze α-1,4-glycosidic bonds, while the so-called iso-amylases (AtISA) and limit dextrinases (AtLDA) cleave α-1,6-glycosidic bonds. Several lines of evidence indicate that the action of β-amylases and iso-amylases contributes mostly to transitory starch degradation in vivo. Mutants deficient either in AtBAM3 (formerly named BMY8) or AtISA3 are impaired in starch degradation resulting in a starch excess and growth retardation phenotype [18, 19]. The impact of α-amylolysis to normal transitory starch breakdown is still not clear. Arabidopsis mutants deficient in all three existing α-amylases (AtAMY1–3) show normal starch degradation [14].
However, the release of branched α-glucans by chloroplastic AtAMY3 contributes to starch degradation in DBE-deficient mutants [18, 20]. Furthermore, the release of glucans from native starch granules by recombinant AtAMY3 in vitro is stimulated by β-amylase [21].
A direct attack of the starch granule by the plastidial phosphorylase (AtPHS1) in vivo and the release of glucosyl residues as glucose-1 phosphate seems to be minor, as the described mutants reveal no or only a small reduction in the starch degradation capability [22, 23]. However, recent findings indicate that AtPHS1 is involved in transitory starch turnover on a so far unknown regulatory level [23].
Interestingly, none of the plastidial amylolytic enzymes act significantly on native starch granules in in vitro experiments. However, amylases considerably hydrolyze starch after disruption of the starch’s semi-crystalline structure [24–27].
Phosphorylation of starch granules
In 1998, Lorberth and colleagues [28] identified a non-amylolytic, starch granule-associated protein in potato, named R1, in which reduction results in a starch excess phenotype in leaves, e.g., the accumulation of high amounts of starch at the end of a normal dark phase because of the decreased rates of leaf starch degradation. In addition, the lowered expression of R1 in these plants is accompanied by a reduction in cold-induced sweeting in tubers [28]. Next to these phenotypical alterations, a reduction in starch’s covalently attached phosphate content is observed in the mutant plants. The existence of phosphoesters in starch, as the only known covalent modification of starch, was discovered in the early 1970s [29, 30]. The amount of starch phosphate differs according to plant species, but is generally low [31]. In Arabidopsis, leaf starch and potato tuber starch about 0.1 and 1 % of the glucosyl residues are phosphorylated, respectively. Independent of starch’s origin, phosphate esters are mainly linked to the amylopectin molecule [32]. In principle, each carbon atom of a glucosyl residue that carries a hydroxyl group can be esterified with a phosphate group. However, only glucose-6 phosphate (G6P) and glucose-3 phosphate (G3P) are found in starch hydrolysates indicating the phosphorylation of the C6 and the C3 hydroxyl group, respectively [30, 33]. In addition, a phosphorylation of the hydroxyl group at the C2 position was described, but so far it was neither detected in Arabidopsis leaf starch nor in potato tuber starch [29, 30].
Transitory starch is continuously phosphorylated during starch synthesis in the light phase, as well as during starch degradation in the dark. However, the starch phosphorylation rate is higher during starch breakdown, as revealed by radioactive labeling experiments using the unicellular green alga Chlamydomonas reinhardtii and 32P-Phosphate [34]. Like higher plants, C. reinhardtii synthesizes and degrades starch in a diurnal rhythm [35]. Moreover, starch is phosphorylated and exogenously supplied phosphate is incorporated into starch at a constant rate when the algae are illuminated. During the first 30 min of darkness, the phosphate incorporation exceeds that of illuminated cells [34]. Likewise, the study shows that phosphorylated glucans are more abundant at the granule surface of potato leaf starch during the beginning of starch mobilization.
Using C. reinhardtii cells, the turnover (the process of starch phosphorylation and dephosphorylation) of the starch-bound phosphate pool was analyzed by 32P-pulse-chase experiments. While the incorporated radioactive phosphate label is stable during 120 min of illumination, in darkened cells the label is reduced by more than 50 % within 20 min after the chase [34]. Higher rates of starch phosphorylation are accompanied by a higher turnover of the phosphate groups indicating a transient esterification [34].
Starch phosphorylating enzymes
The formation and the physiological function of glucan phosphorylation were unclear until Ritte et al. [36] showed that the starch-binding R1 protein from potato catalyzes the glucan phosphorylation in a dikinase reaction type and, therefore, R1 was named glucan, water dikinase (GWD; StGWD from Solanum tuberosum; EC 2.7.9.4). As opposed to various known kinases, dikinases use ATP as a dual phosphate donor and transfer the β- and γ-phosphate groups to two distinct acceptor molecules, a glucan and water.
The Arabidopsis mutant sex1 (starch excess1; At1g10760) that is deficient in a protein homologous to StGWD has a starch excess phenotype in leaves, that is similar to that of transgenic potato plants with reduced StGWD expression, because of impeded starch degradation and an overall reduction in starch phosphate content [37, 38]. Thus, glucan phosphorylation mediated by the non-amylolytic GWD is crucial for proper starch turnover in higher plants. In in vitro assays using native starch granules, the action of recombinant GWD at the granule surface facilitates the release of maltose by plastidial β-amylase and iso-amylase [25].
Using a proteomic approach and reverse genetics, a second starch phosphorylating enzyme, named phosphoglucan, water dikinase (PWD; AtPWD from Arabidopsis thaliana; EC 2.7.9.5; At5g26570), located in the plastids of Arabidopsis plants was identified. PWD mutants have a starch excess phenotype, which, however, is not as severe as in the AtGWD mutant [39, 40]. A gene coding for a third dikinase enzyme (AtGWD2, At4g24450) was also described in Arabidopsis. The recombinant expressed protein is able to phosphorylate glucan substrates in vitro, but unlike mutants deficient in AtGWD and AtPWD, AtGWD2 knock out mutants do not accumulate high amounts of starch nor have a visible growth phenotype compared to wild type (WT) plants. Localization studies reveal an extra plastidial location and the expression of AtGWD2 is in a relatively late stage of plant development. Therefore, a direct access of AtGWD2 to leaf starch granules in vivo and an overall impact on transitory starch metabolism is excluded [41].
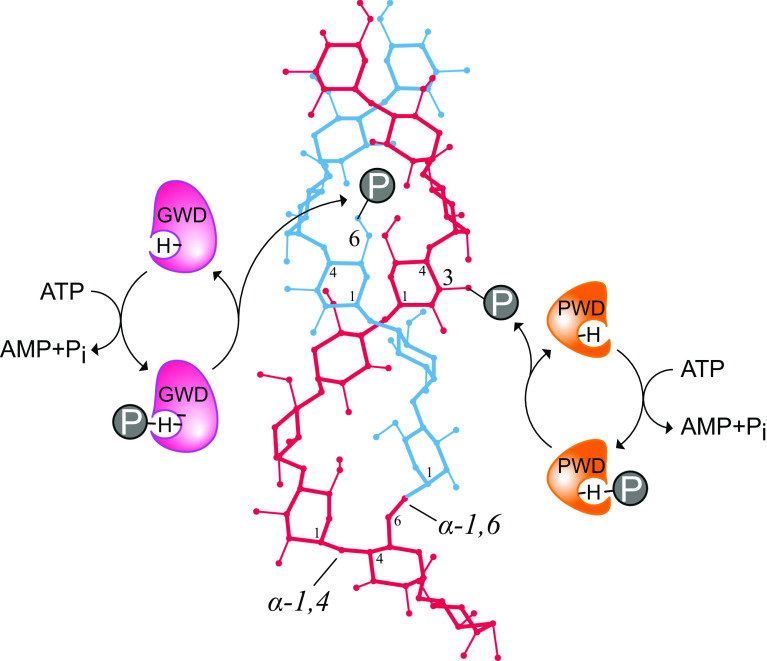
The plastidial dikinases act as monomeric proteins [42]. They share similarities in their amino acid sequences, as well as in the catalyzed reactions. Figure 1 shows the schematic amino acid sequence of mature GWD and PWD including functional domains. The highest homology between both enzymes is found in the most C-terminal region, which closely resembles the ATP-binding domain of bacterial pyruvate, water dikinase (EC 2.7.9.2) and pyruvate, phosphate dikinase (EC 2.7.9.1) [43]. In this regard, the starch phosphorylating enzymes have a second region with high homology to the mentioned bacterial enzymes, the phosphohistidine domain [40, 43]. A conserved histidine residue within this domain is capable of accepting the β-phosphate group of ATP following nucleotide binding and hydrolysis (Fig. 2), which is shown by radioactive labeling experiments using recombinant GWD [36, 43]. Similar to other phosphohistidines as phosphoenzyme intermediates, the phosphoramidate bond is acid labile, but rather stable under alkaline conditions [40, 44]. The γ-phosphate group is transferred to water.
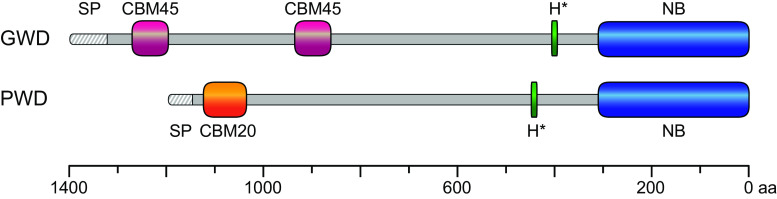
Fig. 1.
Schematic representation of the amino acid sequences of the two plastidial dikinases GWD and PWD from Arabidopsis thaliana including highlighted functional domains. The precursors of both nuclear-encoded dikinase contain an N-terminal signal peptide (SP) for plastid translocation followed by starch-binding domains. In case of GWD, the starch-binding domain is formed by two CBM45s (carbohydrate-binding module; magenta) orientated in tandem domains, which are separated by a 200 amino acid linker. PWD has a single CBM20 (orange) starch-binding domain. The C-terminal part of both sequences shares high homology. It is characterized by a phosphohistidine domain (H*; green) and a nucleotide-binding domain (NB; blue). The unit of the scale at the bottom is amino acids (aa)
Fig. 2.
Schematic illustration of the plastidial dikinases-mediated reactions on α-glucan structures. A left-handed α-1,4-linked glucan chain (red) forms a double helix (upper part) with another glucan chain (blue) connected by a α-1,6-linkage (lower part). Both dikinases act on glucan structures and introduce phosphate esters (P); GWD (magenta) phosphorylates the hydroxyl group at carbon atom 6 while PWD (orange) phosphorylates the C3-hydroxyl group. Before glucan phosphorylation, both enzymes bind and hydrolyze ATP. The γ-phosphate group of ATP is transferred to water and the β-phosphate group to an auto-catalytical histidine residue (H) via a phosphoramidate bond. Subsequently, the β-phosphate is transferred to the glucan substrate
The largest differences in the amino acid sequence of GWD and PWD span the non-catalytic N-terminal region. In case of PWD, the N-terminus contains a single starch-binding domain (SBD) that belongs to the well-characterized carbohydrate-binding module (CBM) family CBM20 [39, 40, 45]. In contrast to PWD, the identity of the N-terminal starch-binding domain of GWD is less pronounced but could be assigned to the recently identified CBM45 family [46, 47]. In particular, two CBM45s orientated in tandem domains and separated by a 200 amino acid linker form the SBD of GWD spanning a sequence of about 500 amino acids. Transient expression of a fusion protein in Arabidopsis containing the CBM45 double module, a YFP, and a signal peptide for chloroplast translocation results in a specific fluorescence signal around the starch granules, indicating the binding of the SBD to native starch [47]. Unfortunately, the biochemical and biophysical characteristics of the CBM double module were very difficult to investigate in in vitro experiments because of rapid aggregation and precipitation of the recombinant protein after expression in E. coli [47]. However, the GWD full-length protein binds to native starch granules in vivo and in vitro [28, 48]. Binding of GWD, as well as of PWD, in vivo is dependent on the metabolic status of the cells. A significantly higher proportion of the dikinases is associated with native leaf starch granules isolated during the dark period than in the light phase of the photoperiod [40, 48]. Moreover, binding of GWD and PWD to native starch granules isolated from the GWD null mutant sex1, that has an overall lowered starch phosphate content, is less compared to sex1 starches that have been prephosphorylated by recombinant GWD [40].
Comparative analysis of the relative amounts of phosphate groups esterified to the C6 or C3 hydroxyl group of glucosyl residues in the starch hydrolysates from Arabidopsis WT and the plastidial dikinase single mutants show that the lack of GWD or PWD causes a reduction of G6P and G3P or G3P alone, respectively [33, 49]. These results indicate that GWD affects both types of phosphate esters, while PWD is mainly responsible for C3 phosphorylation. However, using radioactive labeling experiments, Ritte and co-workers [33] showed that GWD and PWD selectively phosphorylate the C6 and the C3 hydroxyl group of starch’s glucosyl residues, respectively (Fig. 2). The reduction of G6P as well as G3P in the hydrolysate of sex1 starch is explained by the fact that a significant PWD-mediated C3 phosphorylation requires the preceding phosphorylation by GWD in Arabidopsis WT starch [40].
Action of starch-related dikinases on glucan substrates
In contrast to the plastidial amylolytic enzymes, GWD as well as PWD acts significantly on the surface of native starch granules. Nevertheless, the analysis of the physiological function of the dikinase-dependent starch phosphorylation and its stimulating effect on starch hydrolysis catalyzed by AtBAM3 is difficult to determine in vitro using native starch granules. Such an approach is limited because the action of both dikinases is restricted to the granule surface and glucan chains exposed at the surface account only for a minor proportion of the entire granule. Moreover, glucan chains that are phosphorylated by the dikinases remain covalently linked to the insoluble starch particle. Thus, an in vitro system was established using crystalline maltodextrin (MDcryst) as a model substrate for glucan phosphorylating enzyme activity that mimics features of native starches, such as allomorph and crystallinity but omitted branching [50, 51]. Thus, MDcryst has a higher degree of crystallinity and, therefore, recombinant GWD phosphorylates MDcryst with much higher rates than any other native starches tested so far. The incorporation of phosphate esters results in the release of phosphorylated (single, double, and triple phosphorylated glucan chains) as well as neutral maltodextrins from the water-insoluble MDcryst, indicating that the action of GWD disrupts the ordered arrangement of maltodextrins at the particle surface [50].
Likewise to native Arabidopsis WT starch granules, significant phosphorylation of MDcryst by PWD requires the preceding action of GWD [51]. However, in a strict sense the pre-phosphorylation of GWD is not necessary for the action of PWD. It seems like that GWD-dependent phosphorylation alters the granule surface structure, which favors the action of PWD. Thus, PWD phosphorylates also non pre-phosphorylated glucan chains [51]. This is in agreement with the data obtained from labeling experiments using native starch granules isolated from several transgenic potato lines and recombinant PWD. Without any pre-phosphorylation by recombinant GWD, the incorporation of phosphate esters catalyzed by PWD is measured under initial, non-substrate limiting conditions. As a result, there is no relation between the endogenous starch-bound G6P content and the phosphorylation rate of PWD [52]. Interestingly, the presence of small soluble maltodextrins results in a reduction of phosphorylation events on the surface of insoluble glucans. Furthermore, a phosphorylation of soluble glucans is not to observe [51].
It can be concluded that GWD preferentially acts on crystalline surfaces and GWD-mediated phosphorylation enables a phase transition at the granule surface from a solid to a more soluble state enabling a significant amylolysis, which was suggested earlier [25, 53]. Thus, the action of GWD is generally accepted as an initial process during starch degradation [20, 42, 54, 55].
Nevertheless, for normal starch degradation also the removal of the phosphate esters is necessary. Thus, the phosphate esters introduced into starch are, to a significant extent, transient and a starch phosphorylation–dephosphorylation cycle at the granule surface takes place [56]. The genome of Arabidopsis thaliana encodes for three putative starch-related phosphatases located in the plastids, starch excess 4 (SEX4; At3g52180), like-sex-four1 (LSF1; At3g01510), and like-sex-four2 (LSF2; At3g10940). For more information, see the review by Gentry and co-workers [57].
Phenotypical consequences of lacking the starch-related dikinases
As mentioned before, the lack of GWD or PWD results in a starch excess phenotype and retardation in growth, which is more pronounced in case of missing GWD [40]. Thus, the lack of GWD in mature Arabidopsis plants as observed in the null mutant sex1-8 results in a fivefold increased leaf starch content compared to WT plants [26, 37, 40]. In principle, an elevated number of starch granules per chloroplast and/or an increased size of the granules could cause the higher starch amount in the mutants. WT Arabidopsis cells contain 50–100 chloroplasts, which in turn contain several starch granules (5–7 [58]; 4–7 [59]). In mature leaves, there is only little change in the granule number per chloroplast in the diurnal rhythm in WT cells [59]. Changes in starch content seem to be a result of changes in the granule volume rather than a high initiation of newly synthesized granules [59, 60]. Transmission electron microscopy of leaf sections reveals that mature leaves of the sex1-8 mutant contain more starch granules per chloroplast than WT plants. Moreover, isolated native starch granules that are analyzed by scanning electron microscopy (SEM) appear to be bigger in size, which coincides with a higher amount of glucose per granule [26]. Additionally, sex1-8 starch granules are irregular shaped compared to disc-shaped WT granules [26]. This observation prompted to analyze the (surface) properties of isolated sex1-8 starch in more detail.
The chain length distribution (CLD) of α-1,4-glucan chains that are exposed on the surface of native WT starch granules and accessible by plant-encoded amylolytic enzymes, such as iso-amylase, resembles the CLD of the whole granule produced by iso-amylolytic digest of heat-solubilized starch granules. Interestingly, this is not the case for GWD-deficient mutants. Although the internal structure of sex1-8 starch granules is similar to WT, the length as well as the abundance of glucan chains exposed on the granule surface differs in the mutant. The lack of GWD in mature sex1-8 plants results in a shift of the CLD towards shorter glucan chains at the granule surface. In addition, the abundance of glucan chains at the granule surface is increased in the mutant compared to WT, which is indicated by the higher maltose released by β-amylase treatment of native granules [26]. Similar to the Arabidopsis GWD null mutant, the altered surface properties are also present in two partially complemented sex1-8 mutants which have compared to WT an approx. 79 and 93 % lowered GWD protein level, respectively. The higher amount of short glucan chains at the surface of starch granules isolated from GWD-deficient mutants impedes GWD action in vitro. In this sense, GWD-dependent incorporation of starch phosphate monoesters is higher after removing these surface-exposed glucans by amylolytic enzymes. Taken together, the lack of GWD results not only in changes of the starch amount, but also in alterations of granule number, shape, and surface properties. All these alterations are not only restricted to the dark period as they were also observed during the light period [26].
Contribution of glucan phosphorylation to starch synthesis
Although there is a good understanding of the phenotypical alterations caused by GWD deficiency and how glucan phosphorylation facilitates transitory starch degradation mechanistically, several aspects of the dikinases-mediated action remain unclear.
If the observed alterations in mutants lacking GWD are strictly related to the absent of GWD, a function of glucan phosphorylation during the process of starch synthesis has to be predicted.
It is believed that repeating rounds of reduced starch degradation are responsible for the high starch amount in mature GWD-deficient mutants [20]. However, the activities of enzymes, such as starch synthases and branching enzymes, known to be functional during starch synthesis are lowered in GWD-deficient mutants [26]. In addition, Arabidopsis mutants with an up to 70 % lowered GWD protein amount have reduced levels of leaf starch synthesis compared to WT [61]. The comparative analysis of starch synthesis in WT and mutants lacking GWD is hindered by the unequal initial conditions in vivo as mature GWD mutants accumulate up to five times more starch than WT plants. Thus, it is difficult to analyze the direct effects of GWD to starch synthesis in the background of indirect and/or starch breakdown-dependent effects that arise from the diurnal transition of the starch pool.
Skeffington and co-workers [61] tried to uncouple transitory starch synthesis from mobilization in Arabidopsis and measured the rate of starch synthesis in mature inducible GWD-deficient plants having a WT-like starch amount at the beginning of the analysis. No significant difference in the synthesized starch amount is measured in the mutants compared to WT after illuminating the plants following a 60 h dark treatment. The authors conclude that GWD affects starch synthesis more indirectly than directly [61]. In contrast, evidence from in vitro experiments with purified starch granules (isolated from WT and plants with altered GWD expression) and recombinant enzymes suggests that the GWD-mediated glucan phosphorylation of the granule surface can directly influence the elongation of external glucan chains. Thus, the altered starch granule surface properties observed in mutants with reduced expression of GWD impair the elongation of existing glucan chains catalyzed by starch synthase 1 (AtSS1), one of the dominate starch synthesizing activity in Arabidopsis [62], in vitro. In addition, a significant higher amount of glucosyl residues are transferred by AtSS1 to the native starches when the starch granules were pre-phosphorylated by recombinant GWD [26].
These results show that the precise function of the dikinase-mediated phosphorylation during transitory starch synthesis still needs to be defined. Nevertheless, the amount of starch phosphate esters incorporated during starch synthesis is important, at least for the subsequent dark period. This was concluded after analyzing the Arabidopsis WT and the GWD null mutant sex1-8 along with the two partially complemented GWD mutants [63]. When mature light–dark grown WT and mutant plants are exposed to a prolonged darkness of 60 h, the residual GWD activities in the two partially complemented lines are insufficient to degrade starch to WT level [63]. Another indication for a function of starch phosphorylation during starch synthesis is related to potato tuber reserve starch. Thus, in transgenic potato lines with reduced expression of StGWD, small alteration in storage starch metabolism is reported [64, 65]. The antisense lines reveal more but smaller tubers. Furthermore, the amylose content as well as the starch viscosity is affected. Although StGWD transcripts and protein are present in mature tubers, which was shown earlier [28, 66], only recently, the abundance of mRNA coding for the potato homolog of PWD (also named StGWD3) in tuber amyloplasts was confirmed [67]. StPWD is associated with starch granules formed in amyloplasts as indicated by immunochemical localization studies; nonetheless, a functional analysis is still necessary.
However, whether the dikinases have a function during the long time of reserve starch formation remains obscure. Nevertheless, it was shown that allelic variations of the genes encoding StGWD, but also of starch branching enzymes and starch synthase 3, influence the starch phosphorylation in potato tubers [68].
Regulation of dikinase enzyme activity
In the context of enzyme action in vivo, the regulation of the dikinases is of special interest. Nothing is known about the regulation of PWD and only little is known about GWD regulation. Thus, a redox-mediated regulation is reported for StGWD based on the formation of a specific intramolecular disulfide bond between two cysteine residues that are also present in other species, such as Arabidopsis. However, this proposed regulation would result in an active, reduced GWD that has no interaction with the substrate, whereas the oxidized, inactive form is bound to starch granules [66]. Furthermore, transgenic Arabidopsis plants expressing AtGWD containing the Cys mutation that gives constitutive activation have similar starch degradation rates compared to WT plants. Thus, no evidence is present that the redox regulation of GWD is required for either the initiation or the adjustment of the rate of starch degradation. However, a posttranslational level of control is proposed [61].
Interestingly, detailed analysis of the GWD enzymatic steps shows that the γ-phosphate group of ATP is not directly transferred to water. It is proposed that a further, so far unknown, amino acid residue is involved in the transfer of the γ-phosphate group to water releasing orthophosphate. In addition, a transfer of the γ-phosphate to AMP and ADP is observed releasing ADP and ATP, respectively [69]. Therefore, also a posttranslational regulation is discussed to prevent these ‘nonproductive’ reactions.
Genetically modified starch phosphate metabolism in planta
Recent studies highlight the biotechnological potential of alterations in starch phosphorylation of major crop plants and link GWD-mediated activity to biomass production. Several attempts were made to alter the expression of GWD homologs in potato [28, 64, 65], barley (Hordeum vulgare) [70], maize (Zea mays) [71], wheat (Triticum aestivum) [72], and rice (Oryza sativa) [73] and mutant analyses aimed at economically important traits, such as physicochemical properties of starch, starch content, and plant biomass. The above-listed plants accumulate large amounts of reserve starch in their economical important parts, such as tubers and seeds. However, also the starch stored in non-food parts, such as leaves, is of growing interest because of its potential function as an alternative feedstock for ethanol production [74]. The usage of crop residues instead of starchy food parts could reduce the competition between “food, feed, and fuels”. The effects of altered GWD expression to leaf starch content are analyzed in maize and rice. Rice mutants deficient in OsGWD, as well as repression of ZmGWD in maize by RNAi, lead to increased starch accumulation in leaves without any visible difference in vegetative growth [71, 73]. However, in field trials, the overall grain yield is by 30 % lower for rice plants with disrupted OsGWD compared to WT [73]. As starch synthesis in heterotrophic tissue is dependent on the sugars supplied by source organs, such as leaves, throughout the day night cycle and carbon availability at night dependents on the reserves that were stored in the preceding light phase, impaired starch degradation caused by GWD deficiency may lead to lowered export of sugars to heterotrophic tissue and an overall reduction of biomass.
Although starch stored in tubers and seeds represents the major carbohydrate resource of our diet, relatively little is known about glucan phosphorylation, especially, in the starchy endosperm. The phosphate content observed in starch stored in seeds is rather low compared to leaf starch. However, Carciofi and colleagues report about a tenfold increase in grain starch-bound phosphate in barley by heterologous overexpression of StGWD in endosperm amyloplasts [70]. The high starch phosphate content in these transgenic lines results in altered granule morphology and affects the thermal properties of grain starch. The latter are of special interest as many industrial and food applications of native starch require melting of starch, a process in which the crystalline structure of starch is disrupted in the presence of heat. The melting enthalpy of purified grain starch granules from these barley plants, overexpressing StGWD, is lowered compared to WT. The authors conclude that the increased phosphate content of developing endosperm starch enhances hydration of starch glucan chains and thereby reduces crystallinity [70]. These results are in agreement with the observation in potato tubers. Here, melting enthalpy and crystallinity of purified starches are higher if GWD-mediated starch phosphorylation is suppressed [65].
Another recent study highlights the role of GWD in grain starch phosphorylation and metabolism. The endosperm-specific inhibition of GWD homologs in wheat by RNAi decreases the amount of grain starch-bound G6P by up 70 % [72]. Interestingly and in contrast to any other plant species analyzed so far, the downregulation of GWD in transgenic wheat lines causes increase in vegetative biomass and grain yield compared to WT plants [72, 75]. These observations are consistent, both in glasshouse and field trials. However, the molecular mechanisms linking GWD-dependent starch phosphorylation to grain yield need to be discovered. The authors argue that the grain-specific reduction of GWD may increase the sink strength of the endosperm promoting larger grain size [72].
Taken together, these findings show that starch phosphate content can be engineered by altering GWD expression in major crop plants to improve starch content, starch properties, and biomass. Especially, the use of tissue-specific targeting of GWD is highly desirable to affect starch accumulation in leaves or storage organs depending on starch’s applications. Moreover, tissue-specific lack of GWD can avoid reduction of biomass, as retarded growth is mostly observed when GWD is reduced in the entire plant [37].
Future aspects
Despite the discussed increase of knowledge related to the substrates of the dikinases, still much information is missing. Thus, for example it is unclear, if the enzyme acts on the entire starch granule surface consistently, what is the penetration depth of the enzyme, and is the penetration depth also related to the phosphorylation event. Additionally, a functional interaction with known or even so far unknown proteins/enzymes during the action of GWD and PWD on the starch granule is highly likely.
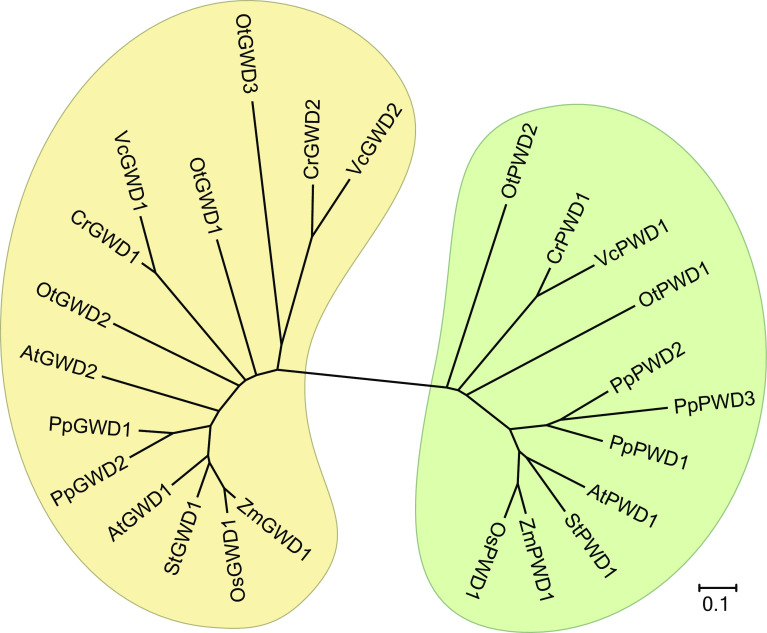
The accelerated availability of whole genome sequences in recent years enables a view beyond higher plants and opens new perspectives in the analysis of glucan phosphorylation. In this regard, the unicellular green alga Ostreococcus tauri and the moss Physcomitrella patens are of interest. Both the genomes of O. tauri and P. patens encode for a high number of five putative starch phosphorylating enzymes [76, 77], which is not the case in other green algae and higher plants (Fig. 3). Up to now, no data exist explaining the function of dikinase multiplicity in both organisms. The characterization of starch-related enzyme activities encoded by multiple genes in other plants, such as Arabidopsis, reveals distinct and, to some extent, redundant functions of enzyme isoforms [15, 78]. Thus, it is most likely that this also holds true for the putative dikinases of O. tauri and P. patens. Elucidating the function of these putative dikinases could enlarge the toolbox of starch-modifying enzymes which can be used in future applications to design starches with novel and desired properties.
Fig. 3.
Dendrogram of glucan phosphorylating dikinases from land plants and homologs from moss and green algae. Phylogenetic analysis was performed according to [79]. The scale bar indicates amino acid substitutions per site. The amino acid sequences used are according to Joint Genome Institute and as follows: At—Arabidopsis thaliana TAIR10 GWD1 (AT1G10760.1), GWD2 (AT4G24450.1), PWD (AT5G26570.1); Cr—Chlamydomonas reinhardtii v5.5 GWD1 (Cre07.g319300.t1.1), GWD2 (Cre07.g332300.t1.2), PWD1 (Cre17.g719900.t1.2); Os—Oryza sativa v7_JGI GWD1 (LOC_Os06g30310.1), PWD1 (LOC_Os12g20150.1); Ot—Ostreococcus tauri v2.0 GWD1 (118), GWD2 (29353), GWD3 (775), PWD1 (18828), PWD2 (10762); Pp—Physcomitrella patens v3.3 GWD1 (Pp3c8_6520V3.1), GWD2 (Pp3c3_11200V3.1), PWD1 (Pp3c18_14870V3.1), PWD2 (Pp3c14_19150V3.1), PWD3 (Pp3c17_18900V3.1); St—Solanum tuberosum v3.4 GWD1 (PGSC0003DMT400019845), PWD1 (PGSC0003DMT400042818); Vc—Volvox carteri v2.1 GWD1 (Vocar.0003s0059.1), GWD2 (Vocar.0009s0098.1), PWD1 (Vocar.0004s0171.1); Zm—Zea mays Ensembl-18 GWD1 (GRMZM2G412611 _T01), PWD1 (GRMZM2G040968_T01)
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG Grant FE 1030/1-1). The authors are grateful to Irina Malinova for help during preparation of the manuscript.
Abbreviations
- AMY
α-Amylase
- BAM, BMY
β-Amylase
- BE
Starch branching enzyme
- CBM
Carbohydrate-binding module
- CLD
Chain length distribution
- DBE
Starch debranching enzyme
- G3P
Glucose-3 phosphate
- G6P
Glucose-6 phosphate
- GWD
Glucan, water dikinase
- ISA
Iso-amylase
- LDA
Limit dextrinase
- LSF
Starch-related phosphatase Like-Sex-Four
- MDcryst
Crystalline maltodextrin
- PHS
Glucan phosphorylase
- PWD
Phosphoglucan, water dikinase
- SBD
Starch-binding domain
- SEM
Scanning electron microscopy
- sex1
Starch excess1 mutant
- SEX4
Starch-related phosphatase 4
- SS
Starch synthase
References
- 1.Buleon A, Colonna P, Planchot V, Ball S. Starch granules: structure and biosynthesis. Int J Biol Macromol. 1998;23(2):85–112. doi: 10.1016/S0141-8130(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 2.Imberty A, Chanzy H, Perez S, Buleon A, Tran V. The double-helical nature of the crystalline part of a-starch. J Mol Biol. 1988;201(2):365–378. doi: 10.1016/0022-2836(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 3.Imberty A, Perez S. A revisit to the three-dimensional structure of b-type starch. Biopolymers. 1988;27(8):1205–1221. doi: 10.1002/bip.360270803. [DOI] [Google Scholar]
- 4.Deschamps P, Colleoni C, Nakamura Y, Suzuki E, Putaux JL, Buleon A, Haebel S, Ritte G, Steup M, Falcon LI, Moreira D, Loffelhardt W, Raj JN, Plancke C, d’Hulst C, Dauvillee D, Ball S. Metabolic symbiosis and the birth of the plant kingdom. Mol Biol Evol. 2008;25(3):536–548. doi: 10.1093/molbev/msm280. [DOI] [PubMed] [Google Scholar]
- 5.Ball S. Evolution of the starch pathway. In: Tetlow IJ, editor. Starch: Origins, structure and metabolism. London: The Society for Experimental Biology; 2012. pp. 29–54. [Google Scholar]
- 6.Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007;30(9):1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 7.Geigenberger P. Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol. 2011;155(4):1566–1577. doi: 10.1104/pp.110.170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu HT, Xu SB, Zheng CH, Wang T. Comparative proteomic study reveals the involvement of diurnal cycle in cell division, enlargement, and starch accumulation in developing endosperm of Oryza sativa . J Proteom Res. 2012;11(1):359–371. doi: 10.1021/pr200779p. [DOI] [PubMed] [Google Scholar]
- 9.Yan D, Duermeyer L, Leoveanu C, Nambara E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014;55(9):1521–1533. doi: 10.1093/pcp/pcu089. [DOI] [PubMed] [Google Scholar]
- 10.Viola R, Pelloux J, van der Ploeg A, Gillespie v, Marquis N, Roberts AG, Hancock RD. Symplastic connection is required for bud outgrowth following dormancy in potato (Solanum tuberosum L.) tubers. Plant Cell Environ. 2007;30(8):973–983. doi: 10.1111/j.1365-3040.2007.01692.x. [DOI] [PubMed] [Google Scholar]
- 11.Sonnewald S, Sonnewald U. Regulation of potato tuber sprouting. Planta. 2014;239(1):27–38. doi: 10.1007/s00425-013-1968-z. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez F, Cejudo FJ. Programmed cell death (pcd): an essential process of cereal seed development and germination. Front Plant Sci. 2014;5:366. doi: 10.3389/fpls.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfister B, Zeeman SC. Formation of starch in plant cells. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu TS, Zeeman SC, Thorneycroft D, Fulton DC, Dunstan H, Lue WL, Hegemann B, Tung SY, Umemoto T, Chapple A, Tsai DL, Wang SM, Smith AM, Chen J, Smith SM. Alpha-amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J Biol Chem. 2005;280(11):9773–9779. doi: 10.1074/jbc.M413638200. [DOI] [PubMed] [Google Scholar]
- 15.Fulton DC, Stettler M, Mettler T, Vaughan CK, Li J, Francisco P, Gil M, Reinhold H, Eicke S, Messerli G, Dorken G, Halliday K, Smith AM, Smith SM, Zeeman SC. Beta-amylase4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. Plant Cell. 2008;20(4):1040–1058. doi: 10.1105/tpc.107.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeeman SC, Umemoto T, Lue WL, Au-Yeung P, Martin C, Smith AM, Chen J. A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both starch and phytoglycogen. Plant Cell. 1998;10(10):1699–1712. doi: 10.1105/tpc.10.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeeman SC, Northrop F, Smith AM, Rees T. A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J. 1998;15(3):357–365. doi: 10.1046/j.1365-313X.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 18.Delatte T, Umhang M, Trevisan M, Eicke S, Thorneycroft D, Smith SM, Zeeman SC. Evidence for distinct mechanisms of starch granule breakdown in plants. J Biol Chem. 2006;281(17):12050–12059. doi: 10.1074/jbc.M513661200. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan F, Guy CL. RNA interference of arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. 2005;44(5):730–743. doi: 10.1111/j.1365-313X.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- 20.Streb S, Zeeman SC. Starch metabolism in Arabidopsis. Arabidopsis Book. 2012;10:e0160. doi: 10.1199/tab.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seung D, Thalmann M, Sparla F, Abou Hachem M, Lee SK, Issakidis-Bourguet E, Svensson B, Zeeman SC, Santelia D. Arabidopsis thaliana AMY3 is a unique redox-regulated chloroplastic alpha-amylase. J Biol Chem. 2013;288(47):33620–33633. doi: 10.1074/jbc.M113.514794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeeman SC, Thorneycroft D, Schupp N, Chapple A, Weck M, Dunstan H, Haldimann P, Bechtold N, Smith AM, Smith SM. Plastidial alpha-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol. 2004;135(2):849–858. doi: 10.1104/pp.103.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinova I, Mahlow S, Alseekh S, Orawetz T, Fernie AR, Baumann O, Steup M, Fettke J. Double knockout mutants of Arabidopsis grown under normal conditions reveal that the plastidial phosphorylase isozyme participates in transitory starch metabolism. Plant Physiol. 2014;164(2):907–921. doi: 10.1104/pp.113.227843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheidig A, Frohlich A, Schulze S, Lloyd JR, Kossmann J. Downregulation of a chloroplast-targeted beta-amylase leads to a starch-excess phenotype in leaves. Plant J. 2002;30(5):581–591. doi: 10.1046/j.1365-313X.2002.01317.x. [DOI] [PubMed] [Google Scholar]
- 25.Edner C, Li J, Albrecht T, Mahlow S, Hejazi M, Hussain H, Kaplan F, Guy C, Smith SM, Steup M, Ritte G. Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial beta-amylases. Plant Physiol. 2007;145(1):17–28. doi: 10.1104/pp.107.104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahlow S, Hejazi M, Kuhnert F, Garz A, Brust H, Baumann O, Fettke J. Phosphorylation of transitory starch by alpha-glucan, water dikinase during starch turnover affects the surface properties and morphology of starch granules. New Phytol. 2014;203(2):495–507. doi: 10.1111/nph.12801. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd JR, Kossmann J, Ritte G. Leaf starch degradation comes out of the shadows. Trends Plant Sci. 2005;10(3):130–137. doi: 10.1016/j.tplants.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Lorberth R, Ritte G, Willmitzer L, Kossmann J. Inhibition of a starch-granule-bound protein leads to modified starch and repression of cold sweetening. Nat Biotechnol. 1998;16(5):473–477. doi: 10.1038/nbt0598-473. [DOI] [PubMed] [Google Scholar]
- 29.Hizukuri S, Tabata S, Nikuni Z. Studies on starch phosphate.1. Estimation of glucose-6-phosphate residues in starch and presence of other bound phosphate(s) Starke. 1970;22(10):338. doi: 10.1002/star.19700221004. [DOI] [Google Scholar]
- 30.Tabata S, Hizukuri S. Starch phosphate. Part 2. Isolation of glucose 3-phosphate and maltose phosphate by acid hydrolysis of potato starch. Starke. 1971;23(8):267. doi: 10.1002/star.19710230803. [DOI] [Google Scholar]
- 31.Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohyd Polym. 2001;45(3):253–267. doi: 10.1016/S0144-8617(00)00260-5. [DOI] [Google Scholar]
- 32.Jane J, Kasemsuwan T, Chen JF, Juliano BO. Phosphorus in rice and other starches. Cereal Foods World. 1996;41(11):827–832. [Google Scholar]
- 33.Ritte G, Heydenreich M, Mahlow S, Haebel S, Kotting O, Steup M. Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett. 2006;580(20):4872–4876. doi: 10.1016/j.febslet.2006.07.085. [DOI] [PubMed] [Google Scholar]
- 34.Ritte G, Scharf A, Eckermann N, Haebel S, Steup M. Phosphorylation of transitory starch is increased during degradation. Plant Physiol. 2004;135(4):2068–2077. doi: 10.1104/pp.104.041301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ral JP, Colleoni C, Wattebled F, Dauvillee D, Nempont C, Deschamps P, Li Z, Morell MK, Chibbar R, Purton S, d’Hulst C, Ball SG. Circadian clock regulation of starch metabolism establishes gbssi as a major contributor to amylopectin synthesis in Chlamydomonas reinhardtii . Plant Physiol. 2006;142(1):305–317. doi: 10.1104/pp.106.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M. The starch-related R1 protein is an alpha-glucan, water dikinase. Proc Natl Acad Sci USA. 2002;99(10):7166–7171. doi: 10.1073/pnas.062053099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu TS, Kofler H, Hausler RE, Hille D, Flugge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, Steup M, Lue WL, Chen J, Weber A. The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell. 2001;13(8):1907–1918. doi: 10.1105/tpc.13.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caspar T, Lin TP, Kakefuda G, Benbow L, Preiss J, Somerville C. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol. 1991;95(4):1181–1188. doi: 10.1104/pp.95.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baunsgaard L, Lutken H, Mikkelsen R, Glaring MA, Pham TT, Blennow A. A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated alpha-glucans and is involved in starch degradation in Arabidopsis . Plant J. 2005;41(4):595–605. doi: 10.1111/j.1365-313X.2004.02322.x. [DOI] [PubMed] [Google Scholar]
- 40.Kotting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol. 2005;137(1):242–252. doi: 10.1104/pp.104.055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glaring MA, Zygadlo A, Thorneycroft D, Schulz A, Smith SM, Blennow A, Baunsgaard L. An extra-plastidial alpha-glucan, water dikinase from Arabidopsis phosphorylates amylopectin in vitro and is not necessary for transient starch degradation. J Exp Bot. 2007;58(14):3949–3960. doi: 10.1093/jxb/erm249. [DOI] [PubMed] [Google Scholar]
- 42.Fettke J, Eckermann N, Kotting O, Ritte G, Steup M. Novel starch-related enzymes and carbohydrates. Cell Mol Biol. 2006;52:OL883–OL904. [PubMed] [Google Scholar]
- 43.Mikkelsen R, Blennow A. Functional domain organization of the potato alpha-glucan, water dikinase (GWD): evidence for separate site catalysis as revealed by limited proteolysis and deletion mutants. Biochem J. 2005;385(Pt 2):355–361. doi: 10.1042/BJ20041119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attwood PV, Piggott MJ, Zu XL, Besant PG. Focus on phosphohistidine. Amino Acids. 2007;32(1):145–156. doi: 10.1007/s00726-006-0443-6. [DOI] [PubMed] [Google Scholar]
- 45.Christiansen C, Abou Hachem M, Janecek S, Vikso-Nielsen A, Blennow A, Svensson B. The carbohydrate-binding module family 20–diversity, structure, and function. FEBS J. 2009;276(18):5006–5029. doi: 10.1111/j.1742-4658.2009.07221.x. [DOI] [PubMed] [Google Scholar]
- 46.Mikkelsen R, Suszkiewicz K, Blennow A. A novel type carbohydrate-binding module identified in alpha-glucan, water dikinases is specific for regulated plastidial starch metabolism. Biochemistry. 2006;45(14):4674–4682. doi: 10.1021/bi051712a. [DOI] [PubMed] [Google Scholar]
- 47.Glaring MA, Baumann MJ, Abou Hachem M, Nakai H, Nakai N, Santelia D, Sigurskjold BW, Zeeman SC, Blennow A, Svensson B. Starch-binding domains in the CBM45 family–low-affinity domains from glucan, water dikinase and alpha-amylase involved in plastidial starch metabolism. FEBS J. 2011;278(7):1175–1185. doi: 10.1111/j.1742-4658.2011.08043.x. [DOI] [PubMed] [Google Scholar]
- 48.Ritte G, Lorberth R, Steup M. Reversible binding of the starch-related R1 protein to the surface of transitory starch granules. Plant J. 2000;21(4):387–391. doi: 10.1046/j.1365-313x.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 49.Haebel S, Hejazi M, Frohberg C, Heydenreich M, Ritte G. Mass spectrometric quantification of the relative amounts of C6 and C3 position phosphorylated glucosyl residues in starch. Anal Biochem. 2008;379(1):73–79. doi: 10.1016/j.ab.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Hejazi M, Fettke J, Haebel S, Edner C, Paris O, Frohberg C, Steup M, Ritte G. Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J. 2008;55(2):323–334. doi: 10.1111/j.1365-313X.2008.03513.x. [DOI] [PubMed] [Google Scholar]
- 51.Hejazi M, Fettke J, Paris O, Steup M. The two plastidial starch-related dikinases sequentially phosphorylate glucosyl residues at the surface of both the a- and b-type allomorphs of crystallized maltodextrins but the mode of action differs. Plant Physiol. 2009;150(2):962–976. doi: 10.1104/pp.109.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fettke J, Hejazi M, Smirnova J, Hochel E, Stage M, Steup M. Eukaryotic starch degradation: integration of plastidial and cytosolic pathways. J Exp Bot. 2009;60(10):2907–2922. doi: 10.1093/jxb/erp054. [DOI] [PubMed] [Google Scholar]
- 53.Blennow A, Engelsen SB. Helix-breaking news: fighting crystalline starch energy deposits in the cell. Trends Plant Sci. 2010;15(4):236–240. doi: 10.1016/j.tplants.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Zeeman SC, Smith SM, Smith AM. The diurnal metabolism of leaf starch. Biochem J. 2007;401(1):13–28. doi: 10.1042/BJ20061393. [DOI] [PubMed] [Google Scholar]
- 55.Orzechowski S. Starch metabolism in leaves. Acta Biochim Pol. 2008;55(3):435–445. [PubMed] [Google Scholar]
- 56.Hejazi M, Fettke J, Steup M (2012) Starch phosphorylation and dephosphorylation: The consecutive action of starch-related dikinases and phosphatases. In: Tetlow IJ (ed) Starch: Origins, structure and metabolism, vol 5. SEB Publishing, London, pp 279–310
- 57.Emanuelle S, Brewer MK, Meekins DA, Gentry MS. Unique carbohydrate binding platforms employed by the glucan phosphatases. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roldan I, Wattebled F, Mercedes Lucas M, Delvalle D, Planchot V, Jimenez S, Perez R, Ball S, D’Hulst C, Merida A. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J. 2007;49(3):492–504. doi: 10.1111/j.1365-313X.2006.02968.x. [DOI] [PubMed] [Google Scholar]
- 59.Crumpton-Taylor M, Grandison S, Png KM, Bushby AJ, Smith AM. Control of starch granule numbers in Arabidopsis chloroplasts. Plant Physiol. 2012;158(2):905–916. doi: 10.1104/pp.111.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibon Y, Blasing OE, Palacios-Rojas N, Pankovic D, Hendriks JH, Fisahn J, Hohne M, Gunther M, Stitt M. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004;39(6):847–862. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 61.Skeffington AW, Graf A, Duxbury Z, Gruissem W, Smith AM. Glucan, water dikinase exerts little control over starch degradation in Arabidopsis leaves at night. Plant Physiol. 2014;165(2):866–879. doi: 10.1104/pp.114.237016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delvalle D, Dumez S, Wattebled F, Roldan I, Planchot V, Berbezy P, Colonna P, Vyas D, Chatterjee M, Ball S, Merida A, D’Hulst C. Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J. 2005;43(3):398–412. doi: 10.1111/j.1365-313X.2005.02462.x. [DOI] [PubMed] [Google Scholar]
- 63.Hejazi M, Mahlow S, Fettke J. The glucan phosphorylation mediated by alpha-glucan, water dikinase (GWD) is also essential in the light phase for a functional transitory starch turn-over. Plant Signal Behav. 2014;9(7):e28892. doi: 10.4161/psb.28892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vikso-Nielsen A, Blennow A, Jorgensen K, Kristensen KH, Jensen A, Moller BL. Structural, physicochemical, and pasting properties of starches from potato plants with repressed R1-gene. Biomacromolecules. 2001;2(3):836–843. doi: 10.1021/bm0155165. [DOI] [PubMed] [Google Scholar]
- 65.Kozlov SS, Blennow A, Krivandin AV, Yuryev VP. Structural and thermodynamic properties of starches extracted from GBSS and GWD suppressed potato lines. Int J Biol Macromol. 2007;40(5):449–460. doi: 10.1016/j.ijbiomac.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Mikkelsen R, Mutenda KE, Mant A, Schurmann P, Blennow A. Alpha-glucan, water dikinase (GWD): a plastidic enzyme with redox-regulated and coordinated catalytic activity and binding affinity. Proc Natl Acad Sci USA. 2005;102(5):1785–1790. doi: 10.1073/pnas.0406674102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orzechowski S, Grabowska A, Sitnicka D, Siminska J, Felus M, Dudkiewicz M, Fudali S, Sobczak M. Analysis of the expression, subcellular and tissue localisation of phosphoglucan, water dikinase (PWD/GWD3) in Solanum tuberosum L.: a bioinformatics approach for the comparative analysis of two alpha-glucan, water dikinases (GWDs) from Solanum tuberosum L. Acta Physiol Plant. 2013;35(2):483–500. doi: 10.1007/s11738-012-1091-y. [DOI] [Google Scholar]
- 68.Carpenter MA, Joyce NI, Genet RA, Cooper RD, Murray SR, Noble AD, Butler RC, Timmerman-Vaughan GM. Starch phosphorylation in potato tubers is influenced by allelic variation in the genes encoding glucan water dikinase, starch branching enzymes I andII, and starch synthase III. Front Plant Sci. 2015;6:143. doi: 10.3389/fpls.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hejazi M, Steup M, Fettke J. The plastidial glucan, water dikinase (GWD) catalyses multiple phosphotransfer reactions. FEBS J. 2012;279(11):1953–1966. doi: 10.1111/j.1742-4658.2012.08576.x. [DOI] [PubMed] [Google Scholar]
- 70.Carciofi M, Shaif SS, Jensen SL, Blennow A, Svensson JT, Vincze E, Hebelstrup KH. Hyperphosphorylation of cereal starch. J Cereal Sci. 2011;54(3):339–346. doi: 10.1016/j.jcs.2011.06.013. [DOI] [Google Scholar]
- 71.Weise SE, Aung K, Jarou ZJ, Mehrshahi P, Li Z, Hardy AC, Carr DJ, Sharkey TD. Engineering starch accumulation by manipulation of phosphate metabolism of starch. Plant Biotechnol J. 2012;10(5):545–554. doi: 10.1111/j.1467-7652.2012.00684.x. [DOI] [PubMed] [Google Scholar]
- 72.Ral JP, Bowerman AF, Li Z, Sirault X, Furbank R, Pritchard JR, Bloemsma M, Cavanagh CR, Howitt CA, Morell MK. Down-regulation of glucan, water-dikinase activity in wheat endosperm increases vegetative biomass and yield. Plant Biotechnol J. 2012;10(7):871–882. doi: 10.1111/j.1467-7652.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 73.Hirose T, Aoki N, Harada Y, Okamura M, Hashida Y, Ohsugi R, Akio M, Hirochika H, Terao T. Disruption of a rice gene for alpha-glucan water dikinase, OsGWD1, leads to hyperaccumulation of starch in leaves but exhibits limited effects on growth. Front Plant Sci. 2013;4:147. doi: 10.3389/fpls.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohlrogge J, Allen D, Berguson B, Dellapenna D, Shachar-Hill Y, Stymne S. Driving on biomass. Science. 2009;324(5930):1019–1020. doi: 10.1126/science.1171740. [DOI] [PubMed] [Google Scholar]
- 75.Bowerman AF, Newberry M, Dielen AS, Whan A, Larroque O, Pritchard J, Gubler F, Howitt CA, Pogson BJ, Morell MK, Ral JP. Suppression of glucan, water dikinase in the endosperm alters wheat grain properties, germination and coleoptile growth. Plant Biotechnol J. 2016;14(1):398–408. doi: 10.1111/pbi.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derelle E, Ferraz C, Rombauts S, Rouze P, Worden AZ, Robbens S, Partensky F, Degroeve S, Echeynie S, Cooke R, Saeys Y, Wuyts J, Jabbari K, Bowler C, Panaud O, Piegu B, Ball SG, Ral JP, Bouget FY, Piganeau G, De Baets B, Picard A, Delseny M, Demaille J, Van de Peer Y, Moreau H. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci USA. 2006;103(31):11647–11652. doi: 10.1073/pnas.0604795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, Tanahashi T, Sakakibara K, Fujita T, Oishi K, Shin IT, Kuroki Y, Toyoda A, Suzuki Y, Hashimoto S, Yamaguchi K, Sugano S, Kohara Y, Fujiyama A, Anterola A, Aoki S, Ashton N, Barbazuk WB, Barker E, Bennetzen JL, Blankenship R, Cho SH, Dutcher SK, Estelle M, Fawcett JA, Gundlach H, Hanada K, Heyl A, Hicks KA, Hughes J, Lohr M, Mayer K, Melkozernov A, Murata T, Nelson DR, Pils B, Prigge M, Reiss B, Renner T, Rombauts S, Rushton PJ, Sanderfoot A, Schween G, Shiu SH, Stueber K, Theodoulou FL, Tu H, Van de Peer Y, Verrier PJ, Waters E, Wood A, Yang L, Cove D, Cuming AC, Hasebe M, Lucas S, Mishler BD, Reski R, Grigoriev IV, Quatrano RS, Boore JL. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319(5859):64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 78.Szydlowski N, Ragel P, Hennen-Bierwagen TA, Planchot V, Myers AM, Merida A, d’Hulst C, Wattebled F. Integrated functions among multiple starch synthases determine both amylopectin chain length and branch linkage location in Arabidopsis leaf starch. J Exp Bot. 2011;62(13):4547–4559. doi: 10.1093/jxb/err172. [DOI] [PubMed] [Google Scholar]
- 79.Beel B, Prager K, Spexard M, Sasso S, Weiss D, Muller N, Heinnickel M, Dewez D, Ikoma D, Grossman AR, Kottke T, Mittag M. A flavin binding cryptochrome photoreceptor responds to both blue and red light in Chlamydomonas reinhardtii . Plant Cell. 2012;24(7):2992–3008. doi: 10.1105/tpc.112.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]