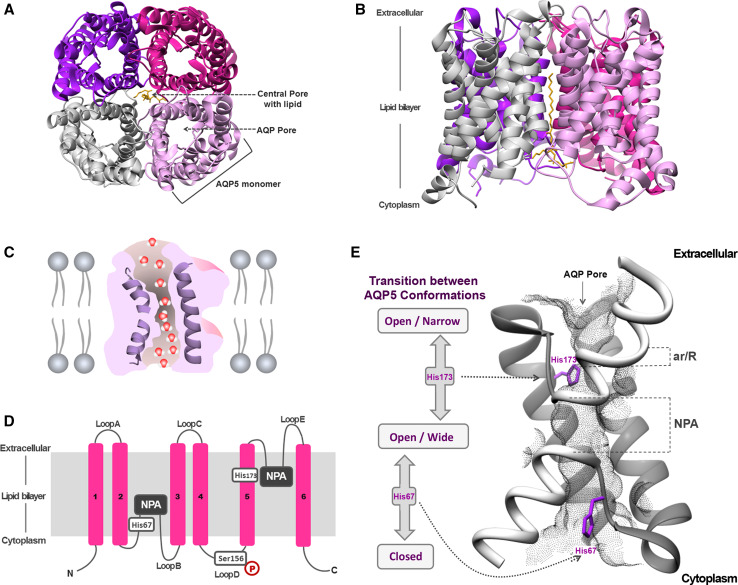
Fig. 4.
Structure of AQP5. a Extracellular and b side views of AQP5 homotetramer showing the central pore with lipid (phosphatidylserine). c Diagram illustrating how water molecules permeate through AQP pore. d Topology map of the basic monomeric AQP5 fold, showing the six transmembrane alpha-helices (1–6) connected by loops (A–E), the conserved asparagine–proline–alanine (NPA) motifs embed in the membrane, the histidine residues involved in gating (His67 and His173) and the serine residue involved in intracellular signaling (Ser156). e Detailed view of AQP5 pore and schematic representation of the proposed AQP5 gating mechanism. The two half-helices are depicted in white and the positioning of ar/R and NPA selectivity filters is indicated. The grey mesh represents the residues lining AQP5 pore. Key histidine residues involved in AQP5 gating are highlighted: His67, which controls the transition between closed and open conformations, and His173, controlling the transition between wide and narrow states. Structures were generated with Chimera (http://www.cgl.ucsf.edu/chimera) and are based on AQP5 X-ray structure (protein data bank code: 3D9S)