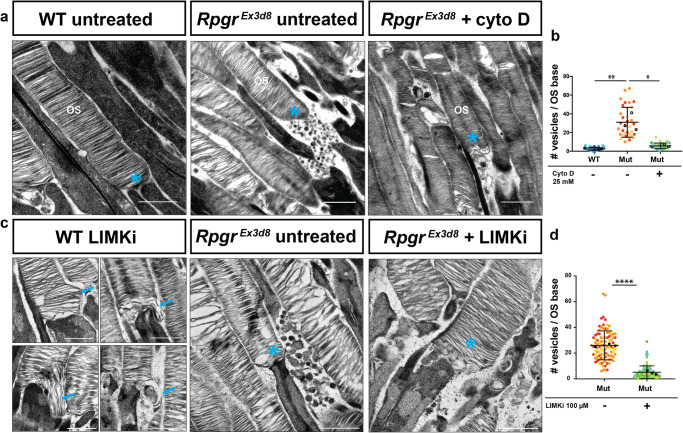
Fig. 6. Targeting actin severing pathways rescues shedding of vesicles from Rpgr-mutant photoreceptors.
a TEM reveals high numbers of vesicles shed from OS bases in RpgrEx3d8 photoreceptors (middle panel) compared to controls (left panel). Shedding of vesicles is reduced upon intravitreal delivery of 25 mM cytochalasin D for 6 h (right panel) (OS = Outer Segment; cyan * = OS base). b Quantification of vesicle shedding at the base of photoreceptors by TEM. (Black symbols = mean number of vesicles at the base of each photoreceptor per experimental animal; N = 3 animals per genotype; error bars represent standard error of the mean; **p = 0.0068, *p = 0.0108; two-tailed, unpaired t-test; error bars denote SEM. Different colours represent measurements from individual mice). c TEM reveals intravitreal delivery of 100 μM LIM kinase inhibitor for 6 h to wild-type retinas leads to elongation of basal discs (blue arrows, left panel). Intravitreal LIMKi delivery to RpgrEx3d8 eyes reduces number of OS shed vesicles (middle and right panels; cyan * = OS base). d Quantification of vesicle shedding by control or LIMKi treatment from TEM images. (Black symbols = mean number of vesicles at OS base of photoreceptors per experimental animal; N = 3 animals per genotype; error bars represent standard error of the mean; ****p ≤ 0.0001; two-tailed, unpaired t-test; error bars denote SEM. Different colours represent measurements from individual mice). (Scale bars; a = 1 µm; c = 500 nm for 4 left panels of wild type treated photoreceptors; 1 µm for middle and right RpgrEx3d8 panels). Source data are provided as a Source Data file.