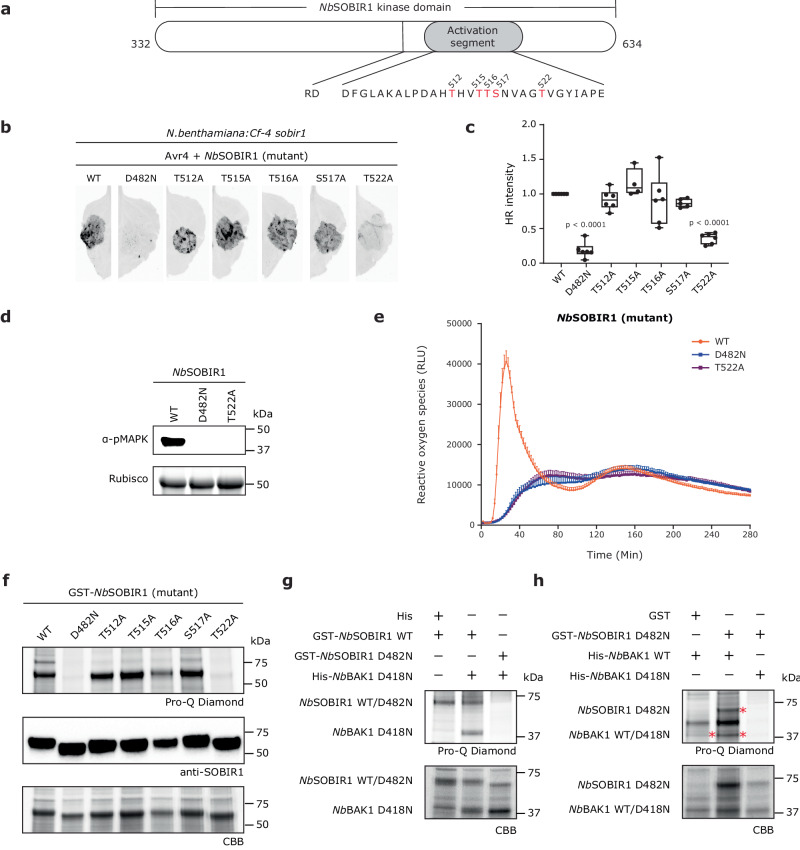
Fig. 1. Activation of the SOBIR1/BAK1-containing immune complex by trans-phosphorylation events between SOBIR1 and BAK1.
a Schematic diagram of the kinase domain of NbSOBIR1. The amino acid sequence of the activation segment of NbSOBIR1 is shown below the diagram. Possible phosphorylation sites are denoted in red. b–e Complementation with NbSOBIR1 T522A fails to restore Avr4/Cf-4-triggered HR (b, c), MAPK activation (d), and ROS burst (e) in N. benthamiana:Cf-4 sobir1 knock-out plants. The development of HR was imaged (b) and quantified (c) at 5 dpi. Statistical significance was determined by a one-way ANOVA/Dunnett’s multiple comparison test, compared with NbSOBIR1 WT. Dots indicate individual values (centre line, median; error bar, minima and maxima; n = 6). Similar to NbSOBIR1 WT, all tested NbSOBIR1 Ser/Thr mutants fully restored the Avr4/Cf-4-triggered ROS production in this complementation study, except for NbSOBIR1 T522A. Only the results for NbSOBIR1 WT, T522A and D482N are shown. ROS production is expressed as relative light units (RLUs), and the data are represented as mean + SEM (n = 12). f Thr522 is required for the intrinsic kinase activity of NbSOBIR1. After SDS-PAGE of the E. coli lysates, the recombinant GST-NbSOBIR1 cytoplasmic kinase domain and its various mutants were stained with Coomassie brilliant blue (CBB) (bottom panel), whereas their accumulation was detected by western blotting (middle panel), and phosphorylation status was determined by performing a Pro-Q Diamond stain (top panel). g NbSOBIR1 WT directly phosphorylates kinase-dead NbBAK1 D418N. h NbBAK1 WT directly phosphorylates kinase-dead NbSOBIR1 D482N. Non-fused GST and His tags served as negative controls. Bands with the expected sizes are indicated with an asterisk. All experiments were repeated at least three times with similar results, and representative results are shown. Source data are provided as a Source Data file.