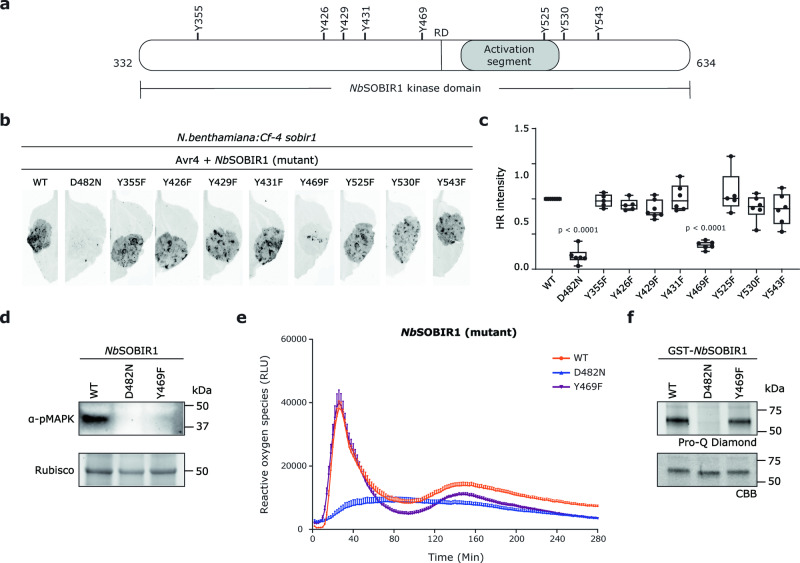
Fig. 2. Tyr469 of the kinase domain of SOBIR1 is crucial for the Avr4/Cf-4-triggered HR and MAPK activation, but not for ROS production and intrinsic kinase activity.
a Schematic diagram of the kinase domain of NbSOBIR1, with the location of the activation segment, the RD motif, and all Tyr (Y) residues indicated. b–d Complementation with NbSOBIR1 Y469F fails to restore Avr4/Cf-4-triggered HR and MAPK activation in N. benthamiana:Cf-4 sobir1 knock-out plants. The development of an HR was imaged (b) and quantified (c) at 5 dpi. Statistical significance was determined by a one-way ANOVA/Dunnett’s multiple comparison test, compared with NbSOBIR1 WT. Dots indicate individual values (centre line, median; error bar, minima and maxima; n = 6). e Transient expression of NbSOBIR1 Y469F restores the Avr4/Cf-4-triggered ROS accumulation in N. benthamiana:Cf-4 sobir1 knock-out plants. Similar to NbSOBIR1 WT, all tested NbSOBIR1 Tyr mutants restored the Avr4/Cf-4-triggered ROS production in this complementation study. Only the results for NbSOBIR1 WT, Y469F and D482N are shown. ROS production is expressed as RLUs, and the data are represented as mean + SEM (n = 8). f NbSOBIR1 Y469F exhibits intrinsic kinase activity. All experiments were repeated at least three times with similar results, and representative results are shown. Source data are provided as a Source Data file.