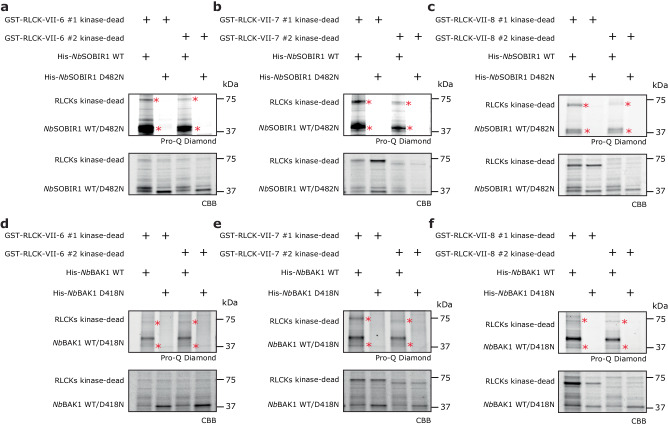
Fig. 5. Members of N. benthamiana RLCK-VII-6, −7, and −8 are directly trans-phosphorylated by both SOBIR1 and BAK1 in vitro.
Two members were randomly selected from RLCK-VII-6, -7, and -8 and their kinase-dead mutants were co-expressed with either the cytoplasmic kinase domain from NbSOBIR1 WT or its D482N kinase-dead mutant (a–c), or with either the cytoplasmic kinase domain from NbBAK1 WT or its D418N kinase-dead mutant (d–f), in E. coli. After SDS-PAGE of the boiled cell lysate, the phosphorylation status of the recombinant proteins was determined by performing a Pro-Q Diamond stain (top panels), while the total proteins were stained with CBB (bottom panels). Bands with the expected sizes are indicated with a red asterisk. Experiments were repeated two times with similar results, and representative results are shown. RLCK-VII-6 #1 kinase-dead, Niben101Scf02460g01004 K110A; RLCK-VII-6 #2 kinase-dead, Niben101Scf06739g05004 K110A; RLCK-VII-7 #1 kinase-dead, Niben101Scf00712g13012 K127A; RLCK-VII-7 #2 kinase-dead, Niben101Scf01176g01025 K119A; RLCK-VII-8 #1 kinase-dead, Niben101Scf00012g00012 K109A; RLCK-VII-8 #2 kinase-dead, Niben101Scf06482g03003 K114A. Source data are provided as a Source Data file.