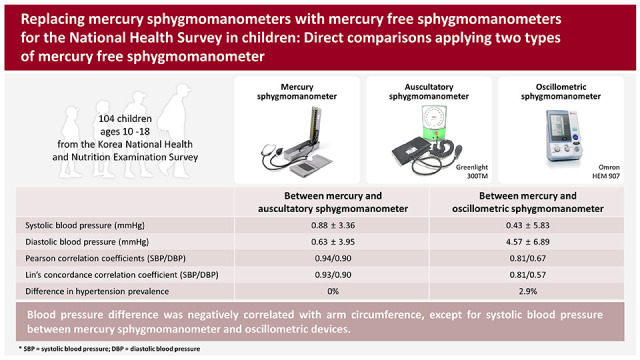
Author's summary
Auscultatory sphygmomanometers are recommended for diagnosing hypertension in children. However, due to environmental concerns, mercury sphygmomanometers (MSs) have been banned, and finding an accurate replacement is necessary. The authors studied the differences and accuracies between MS and mercury-free sphygmomanometers. The mercury-free auscultatory device (AD) measured similar systolic and diastolic blood pressures (DBP) as MS. However, DBP differed between the oscillometric device (OD) and the MS; its absolute error was beyond the acceptable range. The frequency of hypertension also differed between groups. Differences in BP were associated with arm circumference. Therefore, ADs are superior to ODs.
Keywords: Health surveys, Hypertension, Mercury, Sphygmomanometers, Children
Graphical Abstract
Abstract
Background and Objectives
Blood pressure (BP) measurement using an auscultatory sphygmomanometer is recommended for diagnosing hypertension in children. As mercury sphygmomanometers (MSs) are banned owing to environmental concerns, it is crucial to determine the accuracy of mercury-free sphygmomanometers to replace them. We analyzed the accuracy of these devices to guide the National Survey selection.
Methods
BP was measured thrice each with MS, auscultatory device (AD), and oscillometric device (OD) in 104 participants aged 10–18 using the National Survey data. The difference in BP was defined as the difference between MS and other devices. The BP differences, correlations, and influencing factors were analyzed. The frequencies of hypertension were also compared.
Results
Systolic BP (SBP) and diastolic BP (DBP) differences between MS and AD were 0.88±3.36 mmHg and 0.63±3.95 mmHg, and those between MS and OD were 0.43±5.83 mmHg and 4.57±6.89 mmHg, respectively. The absolute error of <10 mmHg for DBP between MS and OD was 76%. The concordance correlation coefficient between MS and AD was 0.94 for SBP and 0.90 for DBP, and 0.81 and 0.67, respectively for MS and OD. Arm circumference negatively correlated with BP differences except for SBP between the MS and OD. The frequency of hypertension was not different between MS and AD but was underestimated by OD.
Conclusions
AD correlated well with MS, while OD did not, especially for DBP. The superiority of AD over OD suggests AD as a possible alternative for MS in the National Survey.
INTRODUCTION
Hypertension is a critical health condition associated with severe cardiovascular complications worldwide. Previously, secondary hypertension was regarded as the primary cause in children; however, the incidence of pediatric essential hypertension has increased, and it has become a crucial health issue with an increasing number of children and adolescents with overweight and obesity.1) Globally, approximately 4.0% of children aged 19 years and younger have blood pressure (BP) measurements categorized as hypertension, and 10% of them have prehypertension.2) Childhood BP is known to be related to hypertension and metabolic syndrome in adulthood.3),4),5) Additionally, cardiovascular complications such as heart failure, stroke, and mortality are associated with both systolic and diastolic hypertension in adults,6),7) which makes accurate measurement and diagnosis of childhood BP essential. For this reason, recent American and European guidelines recommend routine BP measurement from 3 years of age.8),9)
Unlike hypertension in adulthood, hypertension and elevated BP in children and adolescents are defined as BP above the 95th percentile and between the 90th and 95th percentile, respectively, based on the normative BP reference.8),9),10) The normative BP reference values in the Forth Report were established, comprising the BP data derived from approximately 60,000 children using mercury sphygmomanometers (MSs),10) and were updated as the normative BP references by excluding data from children and adolescents with obesity or overweight.8) According to these guidelines, the initial BP measurement may be oscillometric or auscultatory (using a mercury or aneroid sphygmomanometer) but should be confirmed by an auscultatory device.8)
MS was previously the basis of auscultation for BP measurements; however, owing to environmental concerns and the Minamata Convention on Mercury,11) it has been banned for medical uses. Technological advances have led to the development of mercury-free sphygmomanometers, and the accuracy and replaceability of these devices compared to MS is an important issue. The choice of BP measurement method is especially decisive in the National Survey, which is the basis for hypertension policies for screening, diagnosis, and management.
Korea National Health and Nutritional Examination Survey (KNHANES) has collected BP measurement data using MS in children and adolescents aged ten years and older since 1998. However, since MS was banned in Korea in 2020, mercury-free auscultatory (AD) or oscillometric devices (ODs) for BP measurement have been considered as substitutes. Because the choice of BP measurement device is essential in a national survey, evaluating the accuracy of potential devices is required. Several studies have assessed the validity and accuracy of mercury-free devices, mainly among adults,12),13) but few have evaluated these devices in children and adolescents, and no study has evaluated the differences and correlations between the 3 types of devices simultaneously.
The investigators sought to evaluate the differences between MS and mercury-free sphygmomanometers. In addition, we aimed to analyze the risk factors for BP differences and identify changes in the frequency of hypertension according to the devices used, which would help in choosing a more appropriate apparatus to maintain accuracy in the National Survey.
METHODS
Ethical statement
This study was approved by the Institutional Review Board of the Korea Disease Control and Prevention Agency (KDCA) (2018-01-03-P-A).
Study participants
The Association for the Advancement of Medical Instrumentation (AAMI) standards and A Universal Standard for the Validation of Blood Pressure Measuring Devices were used to compare and verify the accuracy of the device.14),15) According to AAMI in the United States, data measured 3 times for 85 subjects are accepted as the primary data for validation.14) When applied to children, 35 subjects from 3 to 12 years of age and 50 subjects aged >12 years are required15); however, in the KNHANES, BP is measured only after age 10. Therefore, to collect a total of 225 (3×85) error values with 3 measurements, 100 subjects were planned to be measured 3 times in consideration of technical issues during measurement owing to patient characteristics.
We consecutively enrolled 104 subjects aged 10–18 years from among those invited to participate in the KNHANES between May and October 2018. These participants met the inclusion criteria, a regular pulse rate during a 15-second examination. Written informed consent for this study was obtained from legal guardians and representatives. Participants who refused to undergo 3 measurements per device were excluded. Data on age, height, weight, arm circumference (AC), and BP were collected.
Device calibration before and after use
The mercury-free electronic sphygmomanometers, including Greenlight 300TM (AD, Accoson, Essex, United Kingdom), and Omron HEM-907 (OD, Omron, Kyoto, Japan), were validated according to the European Society of Hypertension Protocol 2002.16),17) To ensure accuracy before and after use, we conducted calibration procedures on 4 ADs and 4 ODs, comparing them against an MS within a pressure range of 20–270 mmHg. These procedures were carried out in accordance with the British Hypertension Society protocol for evaluating BP measuring devices.18) The calibration process was the same as that in other studies published by the researchers.12)
Blood pressure measurements
BP was measured by 4 trained nurses and verified as professional data collectors for the KNHANES. BP was measured according to previously published standardized KDCA guidelines.12),19) Briefly, BP was measured at intervals of at least 30s, 3 times per device, after 5 minutes of rest between measurements. The participants were seated in a chair with back support and feet flat on the floor, and BP was measured with the right upper arm supported at heart level. The choice of cuff size, arm level, deflation speed, and other quality control issues have been described in a previous study.12) The choice of cuff size was based on the manufacturer’s guidelines for each device. For BP measurement using AD, systolic BP (SBP) was determined by the first Korotkoff sound and diastolic BP (DBP) by the fifth Korotkoff sound (K-5), rounded to the nearest 2 mmHg. The Korotkoff sounds are a series of 5 sounds heard during the measurement of BP. The first sound is a faint, repetitive, sharp tapping sound that is heard 2 or more times in a row and gradually increases in intensity. The fourth Korotkoff sound (K-4) is characterized by a sudden crushing of the sound, and the fifth sound is the point at which all sound disappears. The order of the MS, AD, and OD measurements was randomized to reduce measurement bias. Additionally, the observer was not blinded to the OD readings to facilitate interpretation. All observers in examination centers must undergo the regular “Quality Control and Assurance of Blood Pressure Measurement Program” to minimize inter-observer or intra-observer BP variabilities.20) The detailed description of this has been reported in another report.20)
Blood pressure differences, absolute errors, and blood pressure classification
Based on the recommendation of the KDCA guidelines, the first reading was discarded, and the average value of the 2nd and 3rd measurements was used for the analysis. Thus, the SBP measurement difference (D-SBP) was defined as the average of the 2nd and 3rd SBP measurements obtained from the MS minus the average of the 2nd and 3rd SBP measurements obtained from the AD or OD. The DBP measurement difference (D-DBP) was defined as the average of the 2nd and 3rd DBP measurements obtained from MS minus the average of the 2nd and 3rd DBP measurements from the AD or OD. The absolute error was defined as the absolute value of the difference between MS and other devices in SBP (A-SBP) or DBP (A-DBP). According to the AAMI standards and A Universal Standards, the mean differences and standard deviation of the differences should be 5±8 mmHg (criterion 1),14) and an absolute error (≤10 mmHg) should be at least 85%.15),21)
According to the reference values for Korean children and adolescents,22) BP classification was categorized as normal, elevated BP, and hypertension. Elevated BP was defined as BP between the 90th percentile and 95th percentile by sex, age, and height percentile; hypertension was defined as over the 95th percentile.10) Hypertension was defined based on BP level, without information regarding antihypertensive medication status.
Statistical analysis
The analysis was conducted separately for ages 10–12 (elementary school age, Group 1) and 13–18 (junior to high school age, Group 2) according to A Universal Standard.15) For participant characteristics, categorical variables were summarized as frequencies and percentages, and continuous variables were summarized as means ± standard deviations or as medians and interquartile ranges.
Scatterplots and Bland-Altman plots were drawn for MS and AD, as well as for MS and OD. The differences between MS and AD and between MS and OD were calculated, and their linear relationships were examined using Pearson correlation coefficients (CC). Agreement between MS and AD, as well as between MS and OD, was assessed using Lin’s concordance correlation coefficient (CCC) and Bland–Altman limits of agreement (LOA), which represent the range in which 95% of the measurement differences lie. The percent agreement and kappa values were calculated to assess the level of agreement in the BP classification. Multiple linear regression analysis was performed to determine the factors associated with the BP differences between the devices. To investigate the relationship between AC and BP, participants were categorized into quartiles (Q1–Q4) according to their AC (cm), and the linear trends were tested by applying the contrast of the regression (Q1: 17.3–22.0 cm, Q2: 22.1–24.1cm, Q3: 24.2–26.5 cm, Q4: 26.6–32.3 cm).
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Statistical significance was set at p<0.05.
RESULTS
Baseline characteristics
A total of 104 children participated in the study. The order of BP devices was MS-AD-OD, MS-OD-AD, and OD-MS-AD with 19.2% respectively, followed by AD-OD-MS with 17.3%, AD-MS-OD with 14.4%, and OD-AD-MS with 10.6%. The randomization protocol was implemented with a ratio of 1:2 stratified by age group and 1:1 for sex distribution. Although the OD-AD-MS group was underrepresented, this discrepancy was not statistically significant (Table 1).
Table 1. General characteristics and blood pressure distributions from different devices of study participants.
| Total | Boys | Girls | p value‡ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Overall | Group 1 | Group 2 | Overall | Group 1 | Group 2 | Overall | |||||||
| Number (%) | 36 (34.6) | 68 (65.4) | 104 (100) | 19 (18.3) | 34 (32.7) | 53 (51.0) | 17 (16.3) | 34 (32.7) | 51 (49.0) | 0.0727 | |||||
| p value* | p value* | p value* | p value† | ||||||||||||
| Measurement order, number (%) | |||||||||||||||
| AD-MS-OD | 5 (13.9) | 10 (14.7) | 15 (14.4) | 1.0000 | 3 (15.8) | 5 (14.7) | 8 (15.1) | 0.9995 | 2 (11.8) | 5 (14.7) | 7 (13.7) | 0.9982 | 0.9914 | 1.0000 | |
| AD-OD-MS | 6 (16.7) | 12 (17.6) | 18 (17.3) | 3 (15.8) | 6 (17.6) | 9 (17.0) | 3 (17.6) | 6 (17.6) | 9 (17.6) | 1.0000 | |||||
| MS-AD-OD | 7 (19.4) | 13 (19.1) | 20 (19.2) | 4 (21.1) | 6 (17.6) | 10 (18.9) | 3 (17.6) | 7 (20.6) | 10 (19.6) | 1.0000 | |||||
| MS-OD-AD | 7 (19.4) | 13 (19.1) | 20 (19.2) | 4 (21.1) | 7 (20.6) | 11 (20.8) | 3 (17.6) | 6 (17.6) | 9 (17.6) | 1.0000 | |||||
| OD-AD-MS | 4 (11.1) | 7 (10.3) | 11 (10.6) | 2 (10.5) | 4 (11.8) | 6 (11.3) | 2 (11.8) | 3 (8.8) | 5 (9.8) | 1.0000 | |||||
| OD-MS-AD | 7 (19.4) | 13 (19.1) | 20 (19.2) | 3 (15.8) | 6 (17.6) | 9 (17.0) | 4 (23.5) | 7 (20.6) | 11 (21.6) | ||||||
| p value§ | p value§ | p value§ | p value∥ | ||||||||||||
| Height (cm) | |||||||||||||||
| Mean ± SD | 148.7±8.6 | 166.1±8.4 | 160.1±11.9 | <0.0001 | 149.2±9.9 | 172.4±6.8 | 164.1±13.7 | <0.0001 | 148.0±7.2 | 159.9±4.2 | 156.0±7.7 | <0.0001 | 0.0004 | ||
| Median | 149.2 | 164.3 | 160.0 | 151.2 | 174.6 | 167.0 | 149.1 | 159.6 | 157.8 | ||||||
| Range | 125.9–167.2 | 146.9–183.8 | 125.9–183.8 | 125.9–165.2 | 156.2–183.8 | 125.9–183.8 | 138.3–167.2 | 146.9–171.4 | 138.3–171.4 | ||||||
| Weight (kg) | |||||||||||||||
| Mean ± SD | 44.3±13.4 | 61.4±14.1 | 55.4±16.0 | <0.0001 | 46.6±11.8 | 66.6±15.7 | 59.4±17.3 | <0.0001 | 41.6±14.9 | 56.1±9.9 | 51.3±13.5 | 0.0014 | 0.0089 | ||
| Median | 42.0 | 58.1 | 54.2 | 46.8 | 64.0 | 56.7 | 37.7 | 56.5 | 51.4 | ||||||
| Range | 25.4–94.3 | 38.0–103.6 | 25.4–103.6 | 25.4–74.2 | 46.9–103.6 | 25.4–103.6 | 29.3–94.3 | 38.0–80.4 | 29.3–94.3 | ||||||
| Arm circumference (cm) | |||||||||||||||
| Mean ± SD | 22.8±3.2 | 25.2±3.3 | 24.4±3.5 | 0.0005 | 23.8±3.0 | 26.0±3.5 | 25.2±3.5 | 0.0218 | 21.7±3.1 | 24.4±2.9 | 23.5±3.2 | 0.0038 | 0.0114 | ||
| Median | 22.7 | 25.3 | 24.2 | 24.2 | 25.6 | 24.8 | 21.0 | 24.9 | 23.7 | ||||||
| Range | 17.3–31.2 | 18.5–32.3 | 17.3–32.3 | 17.3–29.7 | 20.0–32.3 | 17.3–32.3 | 18.5–31.2 | 18.5–30.5 | 18.5–31.2 | ||||||
| Systolic blood pressure (mmHg) | |||||||||||||||
| MS | Mean ± SD | 105.2±9.5 | 108.0±9.0 | 107.0±9.2 | 0.1492 | 107.4±11.3 | 110.4±9.0 | 109.3±9.9 | 0.2862 | 102.8±6.7 | 105.5±8.4 | 104.6±7.9 | 0.2528 | 0.0088 | |
| Median | 105.0 | 107.0 | 106.0 | 109.0 | 109.5 | 109.0 | 103.0 | 104.0 | 103.0 | ||||||
| Range | 85.0–131.0 | 89.0–131.0 | 85.0–131.0 | 85.0–131.0 | 93.0–131.0 | 85.0–131.0 | 92.0–112.0 | 89.0–124.0 | 89.0–124.0 | ||||||
| AD | Mean ± SD | 103.8±9.7 | 107.4±9.5 | 106.1±9.7 | 0.0666 | 105.8±11.0 | 109.7±9.5 | 108.3±10.2 | 0.1772 | 101.5±7.8 | 105.1±9.0 | 103.9±8.7 | 0.1650 | 0.0188 | |
| Median | 103.5 | 105.0 | 105.0 | 104.0 | 107.0 | 106.0 | 102.0 | 103.0 | 103.0 | ||||||
| Range | 85.0–125.0 | 92.0–132.0 | 85.0–132.0 | 85.0–125.0 | 96.0–132.0 | 85.0–132.0 | 85.0–112.0 | 92.0–126.0 | 85.0–126.0 | ||||||
| OD | Mean ± SD | 102.6±8.6 | 108.7±9.7 | 106.6±9.8 | 0.0021 | 102.1±9.5 | 111.4±9.2 | 108.1±10.3 | 0.0009 | 103.3±7.8 | 106.0±9.6 | 105.1±9.1 | 0.3146 | 0.1214 | |
| Median | 102.3 | 108.8 | 105.0 | 101.5 | 111.3 | 107.0 | 104.0 | 103.3 | 103.5 | ||||||
| Range | 78.5–121.0 | 87.0–131.0 | 78.5–131.0 | 78.5–121.0 | 92.5–131.0 | 78.5–131.0 | 89.0–113.0 | 87.0–127.5 | 87.0–127.5 | ||||||
| Diastolic blood pressure (mmHg) | |||||||||||||||
| MS | Mean ± SD | 64.6±8.2 | 65.6±9.4 | 65.3±9.0 | 0.5694 | 64.9±7.9 | 65.8±9.8 | 65.5±9.1 | 0.7250 | 64.2±8.8 | 65.4±9.2 | 65.0±9.0 | 0.6481 | 0.7826 | |
| Median | 64.5 | 66.0 | 65.0 | 66.0 | 65.5 | 66.0 | 64.0 | 66.0 | 65.0 | ||||||
| Range | 45.0–86.0 | 45.0–92.0 | 45.0–92.0 | 47.0–80.0 | 45.0–92.0 | 45.0–92.0 | 45.0–86.0 | 46.0–82.0 | 45.0–86.0 | ||||||
| AD | Mean ± SD | 63.0±7.2 | 65.5±9.4 | 64.6±8.7 | 0.1767 | 63.9±8.1 | 65.4±9.7 | 64.9±9.1 | 0.5868 | 62.0±6.1 | 65.5±9.2 | 64.4±8.4 | 0.1572 | 0.7693 | |
| Median | 62.0 | 66.0 | 64.0 | 62.0 | 66.5 | 64.0 | 62.0 | 66.0 | 64.0 | ||||||
| Range | 45.0–81.0 | 45.0–93.0 | 45.0–93.0 | 45.0–81.0 | 45.0–93.0 | 45.0–93.0 | 51.0–72.0 | 46.0–86.0 | 46.0–86.0 | ||||||
| OD | Mean ± SD | 60.8±6.7 | 60.6±7.9 | 60.7±7.5 | 0.9144 | 61.6±7.6 | 60.2±7.9 | 60.7±7.7 | 0.5195 | 59.9±5.7 | 61.1±8.0 | 60.7±7.3 | 0.5819 | 0.9962 | |
| Median | 60.8 | 60.5 | 60.5 | 62.0 | 59.3 | 61.5 | 60.0 | 61.3 | 60.0 | ||||||
| Range | 48.0–77.0 | 45.5–85.5 | 45.5–85.5 | 48.0–77.0 | 45.5–76.0 | 45.5–77.0 | 51.5–72.0 | 45.5–85.5 | 45.5–85.5 | ||||||
Group 1: 10–12 years old; Group 2: 13–18 years old.
AD = auscultatory device (Greenlight 300TM); MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907); SD = standard deviation.
*The p value with χ2 test for measurement order and age group distribution.
†The p value with χ2 test for measurement order and sex distribution.
‡The p value with Fisher’s exact test applied if the value of the cell is less than or equal to 5, and χ2 test if the value is greater than or equal to 5, to test for differences in the distribution by sex and age group.
§The p value with independent t-test for mean comparison by age group.
∥The p value with independent t-test for mean comparison by sex group.
Of the 36 participants aged 10–12 (Group 1), 19 (52.8%) were boys, and of the 68 participants aged 13–18 (Group 2), 34 (50.0%) were boys (Table 1). Height, weight, and AC were greater in Group 2 than in Group 1. When we analyzed AC by sex, we found that the average AC for girls was significantly smaller than for boys in Group 1 (21.7±3.1 vs. 23.8±3.0 cm, p=0.0307). In Group 2, girls had an AC 1.6 cm smaller than that of boys, but the difference was not significant (p=0.0871).
For all 3 devices, the SBP and DBP of Group 2 were greater than those of Group 1 except for the DBP measured using OD (Table 1). The difference in SBP between the groups was 2.8 mmHg when measured using MS, 3.6 mmHg using AD, and 6.1 mmHg using OD. However, the differences between the age groups were not statistically significant except for OD (p=0.0021). Likewise, the average difference in DBP between Groups 1 and 2 was insignificant (Table 1). Regarding BP differences between sexes, SBP was significantly higher in boys with MS (p=0.0088) or AD (p=0.0188), but not statistically significant with OD (p=0.1214). As for DBP, there was no significant difference between the sexes (Table 1).
Blood pressure differences and absolute errors between mercury sphygmomanometer and electric devices
Overall, the SBP and DBP measured by MS were the highest among the 3 devices. The D-SBP and D-DBP between MS and AD were 0.88±3.36 mmHg and 0.63±3.95 mmHg, and those between MS and OD were 0.43±5.83 mmHg and 4.57±6.89 mmHg, respectively, which meet AAMI standards (Table 2). When compared by age, the D-SBP or D-DBP between MS and AD or MS and OD were more prominent in Group 1 than in Group 2, except for the D-DBP between MS and OD.
Table 2. Differences and correlations between MS and electronic devices.
| Number | BP | MS-AD | MS-OD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference | CC | CCC | Difference | CC | CCC | ||||||
| Mean ± SD | r | p value | ρ (95% CI) | Mean ± SD | r | p value | ρ (95% CI) | ||||
| Overall | 104 | SBP | 0.88±3.36 | 0.94 | <0.001 | 0.93 (0.90–0.95) | 0.43±5.83 | 0.81 | <0.001 | 0.81 (0.73–0.87) | |
| DBP | 0.63±3.95 | 0.90 | <0.001 | 0.90 (0.85–0.93) | 4.57±6.89 | 0.67 | <0.001 | 0.57 (0.44–0.67) | |||
| Sex | |||||||||||
| Boy | 53 | SBP | 1.02±3.02 | 0.96 | <0.001 | 0.95 (0.91–0.97) | 1.28±6.46 | 0.79 | <0.001 | 0.79 (0.66–0.87) | |
| DBP | 0.62±3.82 | 0.91 | <0.001 | 0.91 (0.85–0.95) | 4.81±6.53 | 0.71 | <0.001 | 0.60 (0.43–0.73) | |||
| Girl | 51 | SBP | 0.73±3.71 | 0.91 | <0.001 | 0.90 (0.83–0.94) | −0.46±5.01 | 0.83 | <0.001 | 0.83 (0.71–0.90) | |
| DBP | 0.64±4.11 | 0.89 | <0.001 | 0.89 (0.81–0.93) | 4.31±7.30 | 0.62 | <0.001 | 0.53 (0.33–0.58) | |||
| Age group | |||||||||||
| Group 1 | 36 | SBP | 1.47±4.03 | 0.91 | <0.001 | 0.90 (0.81–0.95) | 2.6±6.51 | 0.75 | <0.001 | 0.72 (0.51–0.84) | |
| DBP | 1.53±4.55 | 0.83 | <0.001 | 0.81 (0.66–0.90) | 3.76±5.53 | 0.74 | <0.001 | 0.65 (0.44–0.79) | |||
| Group 2 | 68 | SBP | 0.56±2.93 | 0.95 | <0.001 | 0.95 (0.92–0.97) | −0.72±5.13 | 0.85 | <0.001 | 0.85 (0.76–0.90) | |
| DBP | 0.15±3.53 | 0.93 | <0.001 | 0.93 (0.89–0.96) | 4.99±7.51 | 0.64 | <0.001 | 0.54 (0.37–0.67) | |||
Group 1: 10–12 years old; Group 2: 13–18 years old.
AD = auscultatory device (Greenlight 300TM); BP = blood pressure; CC = Pearson’s correlation coefficient; CCC = Lin’s concordance correlation coefficient; CI = confidence interval; DBP = diastolic blood pressure; MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907); SBP = systolic blood pressure; SD = standard deviation.
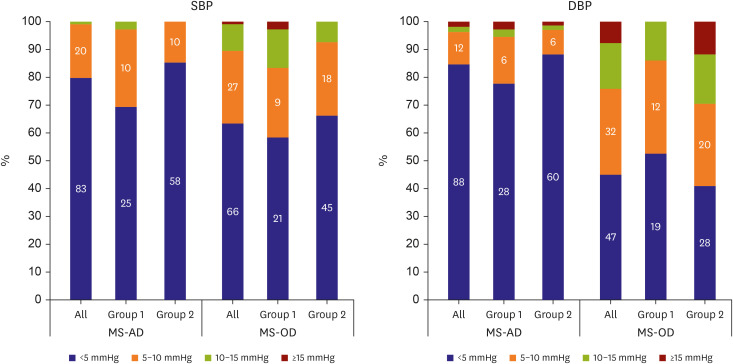
The cumulative percentages of A-SBP and A-DBP <5 mmHg between the MS and AD were 79.8% and 84.6%, respectively, and those of A-SBP <10 mmHg were 99.0% and 96.1%, respectively (Figure 1). In contrast, the A-SBP and A-DBP <5 mmHg between MS and OD were 63.5% and 45.2%, respectively, and those of <10 mmHg were 89.5% and 76.0%, respectively, which are below the AAMI standards for A-DBP. However, A-SBP and A-DBP <10 mmHg between MS and OD in Group 1 were 83.3% and 86.1%, respectively, and those <10 mmHg in Group 2 were 92.7% and 70.6%, respectively, showing different distributions according to age group.
Figure 1. Distribution of the absolute error. The graphs display the number of patients. Group 1: 10–12 years old; Group 2: 13–18 years old.
AD = auscultatory device (Greenlight 300TM); DBP = diastolic blood pressure; MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907); SBP = systolic blood pressure.
Correlation of blood pressure between mercury sphygmomanometer and electric devices
The CC for SBP was 0.94 between MS and AD, and 0.81 between MS and OD, and CCC was 0.93 and 0.81, respectively, indicating a better correlation between MS and AD than MS and OD (Table 2). The correlation of DBP between MS and OD was significantly lower. The CC and CCC for DBP were 0.90 and 0.90 between MS and AD, and 0.67 and 0.57 between MS and OD. In the subgroup analysis, the correlation between MS and OD was weaker than that between MS and AD in all subgroups. Additionally, compared with SBP, CC and CCC were much lower in DBP in all subgroups, especially in DBP between MS and OD.
The scatter plots, CC, and CCC values between MS and AD and between MS and OD for SBP and DBP are shown in Figure 2. Subgroup analysis by age and sex were performed and are shown separately in each figure (Supplementary Figure 1). Compared to CC and CCC in SBP or DBP between MS and AD, those between MS and OD were lower for every age and sex subgroup. The differences between MS and OD were more pronounced in the DBP of girls than in boys, and this trend, according to CCC, was found to be consistent among younger age groups relative to adolescents (Group 1 boys vs. girls, 0.79 vs. 0.48; Group 2 boys vs. girls, 0.54 vs. 0.54). However, despite this decrease, a moderate to good linear relationship and reproducibility were still observed.
Figure 2. The scatter plots and correlations between MS and AD, as well as between MS and OD for SBP and DBP according to sex.
AD = auscultatory device (Greenlight 300TM); CCC = Lin’s concordance correlation coefficient; DBP = diastolic blood pressure; MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907); SBP = systolic blood pressure.
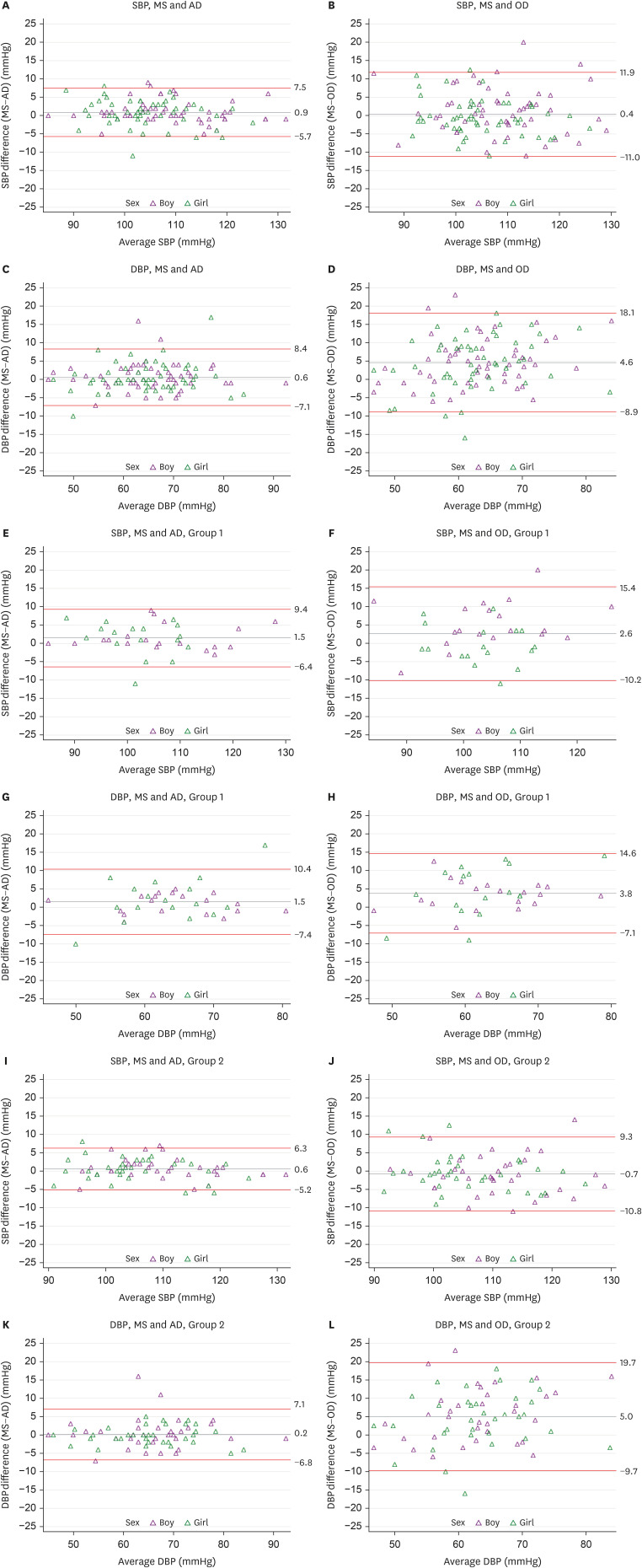
Figure 3 shows the Bland-Altman plot depicting the agreement between MS and AD, as well as between MS and OD. The plots were stratified according to age and sex. The LOA between MS and AD, encompassing 95% of the observations, was determined to be −5.7 mmHg to 7.5 mmHg for SBP and −7.1 mmHg to 8.4 mmHg for DBP among the overall study population. In the MS and OD comparison case, the LOA was observed to be −11.0 mmHg to 11.9 mmHg for SBP and −8.9 mmHg to 18.1 mmHg for DBP. Consistent with the CC and CCC analysis findings, the LOA width was more significant when comparing MS and OD than when comparing MS and AD. Furthermore, the LOA was generally wider for DBP than for SBP and wider in Group 1 than in Group 2.
Figure 3. The Bland-Altman plots between MS and AD, as well as between MS and OD for SBP and DBP according to sex and age groups. Group 1: 10–12 years old; Group 2: 13–18 years old.
AD = auscultatory device (Greenlight 300TM); DBP = diastolic blood pressure; MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907); SBP = systolic blood pressure.
Risk factors for blood pressure differences and absolute errors
Among age, sex, height, and AC, AC was found to have a negative correlation with D-SBP (β=−0.37, p=0.0047) and D-DBP (β=−0.29, p=0.0298) between MS and AD. Likewise, AC was negatively associated with D-DBP between MS and OD (β=−0.65, p=0.0010); however, no factors were related to D-SBP between MS and OD (Table 3). Only the MS-OD-AD sequence had a significant effect on the D-DBP between MS and OD in the BP sequences, while the other sequences did not show any statistical significance.
Table 3. Beta coefficients for a multivariate analysis that takes into account the order of blood pressure measurements.
| MS-AD | MS-OD | |||||
|---|---|---|---|---|---|---|
| β | p value | β | p value | |||
| Systolic blood pressure | ||||||
| Intercept | 1.96 | 0.7390 | −4.36 | 0.6580 | ||
| MS (mmHg) | 0.08 | 0.0831 | 0.13 | 0.0830 | ||
| Age (year) | 0.08 | 0.6616 | −0.45 | 0.1679 | ||
| Boys | 0.63 | 0.4138 | 1.07 | 0.4047 | ||
| Height (cm) | −0.01 | 0.8537 | −0.08 | 0.2962 | ||
| AC (cm) | −0.37 | 0.0047 | 0.33 | 0.1329 | ||
| Measurement order | ||||||
| AD-MS-OD | −0.80 | 0.4864 | 0.12 | 0.9505 | ||
| AD-OD-MS | −0.59 | 0.5941 | 3.07 | 0.0969 | ||
| MS-AD-OD | −0.30 | 0.7775 | 0.23 | 0.8977 | ||
| MS-OD-AD | −0.13 | 0.9038 | 1.83 | 0.3204 | ||
| OD-AD-MS | −0.43 | 0.7337 | −0.30 | 0.8891 | ||
| OD-MS-AD | Ref. | Ref. | ||||
| R square | 0.10 | 0.17 | ||||
| Diastolic blood pressure | ||||||
| Intercept | −3.08 | 0.6069 | −26.78 | 0.0025 | ||
| MS (mmHg) | 0.16 | 0.0002 | 0.49 | <0.001 | ||
| Age (year) | −0.39 | 0.0614 | 0.19 | 0.5171 | ||
| Boys | 0.03 | 0.9744 | 0.73 | 0.5410 | ||
| Height (cm) | 0.03 | 0.5523 | 0.07 | 0.3082 | ||
| AC (cm) | −0.29 | 0.0298 | −0.65 | 0.0010 | ||
| Measurement order | ||||||
| AD-MS-OD | 0.37 | 0.7689 | −0.18 | 0.9184 | ||
| AD-OD-MS | 1.26 | 0.2966 | 2.53 | 0.1490 | ||
| MS-AD-OD | 0.98 | 0.3963 | −1.03 | 0.5389 | ||
| MS-OD-AD | 2.06 | 0.0803 | 3.46 | 0.0430 | ||
| OD-AD-MS | 1.03 | 0.4536 | −0.03 | 0.9896 | ||
| OD-MS-AD | Ref. | Ref. | ||||
| R square | 0.2335 | 0.4759 | ||||
All models were adjusted for measurement order.
AC = arm circumference; AD = auscultatory device (Greenlight 300TM); MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907).
The correlation between blood pressure difference and arm circumference
To investigate the relationship between AC and BP, we divided the participants into 4 groups according to their AC (Table 4). Overall, when looking at the difference between MS and AD, the differences in both SBP and DBP were the largest in the group with the lowest AC (Q1), and they were found to decrease as AC increased, without statistical significance for DBP. In contrast, when looking at the difference between MS and OD, both SBP and DBP showed the highest difference in Q3 and the smallest difference in Q4. However, in sub-analysis according to sex, there were no consistent pattern differences.
Table 4. Distributions of blood pressure difference according to the arm circumference quartile.
| AC quartile | p for trend * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||||||||
| AC (cm) | 17.3–22.0 (20.14±1.45) | 22.1–24.1 (22.87±1.05) | 24.2–26.5 (25.42±1.08) | 26.6–32.3 (28.70±2.01) | |||||||
| BP difference | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Overall | |||||||||||
| MS-AD | |||||||||||
| SBP (mmHg) | 1.94 | (2.97) | 1.50 | (4.20) | 0.73 | (2.99) | −0.56 | (2.65) | 0.0257 | ||
| DBP (mmHg) | 1.33 | (2.96) | 1.26 | (4.76) | 1.12 | (4.55) | −1.09 | (2.67) | 0.1811 | ||
| MS-OD | |||||||||||
| SBP (mmHg) | −0.92 | (4.59) | 1.15 | (6.02) | 1.60 | (5.34) | −0.26 | (6.98) | 0.7342 | ||
| DBP (mmHg) | 4.94 | (7.03) | 5.20 | (5.80) | 5.29 | (7.30) | 2.91 | (7.44) | 0.3124 | ||
| Boys | |||||||||||
| MS-AD | |||||||||||
| SBP (mmHg) | 1.67 | (2.93) | 3.00 | (2.94) | −0.57 | (2.98) | −0.08 | (1.93) | 0.3062 | ||
| DBP (mmHg) | 1.75 | (2.22) | 0.57 | (2.38) | 1.79 | (5.82) | −1.62 | (2.75) | 0.2820 | ||
| MS-OD | |||||||||||
| SBP (mmHg) | −0.63 | (5.58) | 3.11 | (5.18) | 1.36 | (5.69) | 0.92 | (9.02) | 0.7566 | ||
| DBP (mmHg) | 6.08 | (6.91) | 5.64 | (4.58) | 4.64 | (7.98) | 2.92 | (6.54) | 0.0354 | ||
| Girls | |||||||||||
| MS-AD | |||||||||||
| SBP (mmHg) | 2.21 | (3.11) | −0.12 | (4.85) | 2.25 | (2.26) | −1.00 | (3.19) | 0.4321 | ||
| DBP (mmHg) | 0.92 | (3.60) | 2.00 | (6.47) | 0.33 | (2.42) | −0.61 | (2.59) | 0.2607 | ||
| MS-OD | |||||||||||
| SBP (mmHg) | −1.21 | (3.56) | −0.96 | (6.35) | 1.88 | (5.14) | −1.36 | (4.43) | 0.7993 | ||
| DBP (mmHg) | 3.79 | (7.27) | 4.73 | (7.05) | 6.04 | (6.68) | 2.89 | (8.43) | 0.8668 | ||
Arm circumferences are presented as (range, mean±SD).
AC = arm circumference; AD = auscultatory device (Greenlight 300TM); BP=blood pressure; DBP = diastolic blood pressure; MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907); SBP = systolic blood pressure; SD = standard deviation.
*The linear trend was tested by applying the contrast of the regression.
Difference in hypertension prevalence
Among the 104 children who participated in the study, 5 (4.8%) were diagnosed with hypertension, while 14 (13.5%) had elevated BP or hypertension (EBP + HTN) under MS BP measurement. The prevalence of hypertension remained unchanged when measured using the AD, and the number of individuals classified as having EBP + HTN decreased to 11 (10.6%). When measured using OD, the number of hypertension diagnoses decreased to 2 (1.9%), and that of EBP + HTN decreased to nine (8.7%), without statistical significance (Table 5).
Table 5. Prevalences of elevated blood pressure and hypertension by different devices.
| Total | Boy | Girl | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtotal (n=104) | Group 1 (n=36) | Group 2 (n=68) | Subtotal (n=53) | Group 1 (n=19) | Group 2 (n=34) | Subtotal (n=51) | Group 1 (n=17) | Group 2 (n=34) | ||||
| MS | ||||||||||||
| EBP + HTN | 14 (13.5) | 7 (19.4) | 7 (10.3) | 8 (15.1) | 5 (26.3) | 3 (8.8) | 6 (11.8) | 2 (11.8) | 4 (11.8) | |||
| HTN | 5 (4.8) | 3 (8.3) | 2 (2.9) | 3 (5.7) | 2 (10.5) | 1 (2.9) | 2 (3.9) | 1 (5.9) | 1 (2.9) | |||
| AD | ||||||||||||
| EBP + HTN | 11 (10.6) | 4 (11.1) | 7 (10.3) | 7 (13.2) | 4 (21.1) | 3 (8.8) | 4 (7.8) | 0 (0.0) | 4 (11.8) | |||
| HTN | 5 (4.8) | 1 (2.8) | 4 (5.9) | 2 (3.8) | 1 (5.3) | 1 (2.9) | 3 (5.9) | 0 (0.0) | 3 (8.8) | |||
| OD | ||||||||||||
| EBP + HTN | 9 (8.7) | 1 (2.8) | 8 (11.8) | 4 (7.6) | 1 (5.3) | 3 (8.8) | 5 (9.8) | 0 (0.0) | 5 (14.7) | |||
| HTN | 2 (1.9) | 0 (0.0) | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.9) | 0 (0.0) | 2 (5.9) | |||
| Differences in hypertension prevalence | ||||||||||||
| MS vs. AD | ||||||||||||
| EBP + HTN | ||||||||||||
| Difference (%) | 2.9 | 8.3 | 0.0 | 1.9 | 5.3 | 0.0 | 3.9 | 11.8 | 0.0 | |||
| p value | 0.1797 | 0.0833 | 1.0000 | 0.3173 | 0.3173 | 1.0000 | 0.3173 | - | 1.0000 | |||
| HTN | ||||||||||||
| Difference (%) | 0.0 | 5.6 | −2.9 | 1.9 | 5.3 | 0.0 | −2.0 | 5.9 | −5.9 | |||
| p value | 1.0000 | 0.1573 | 0.1573 | 0.3173 | 0.3173 | 1.0000 | 0.5637 | - | 0.1573 | |||
| MS vs. OD | ||||||||||||
| EBP + HTN | ||||||||||||
| Difference (%) | 4.8 | 16.7 | −1.5 | 7.5 | 21.1 | 0.0 | 2.0 | 11.8 | −2.9 | |||
| p value | 0.1317 | 0.0143 | 0.6547 | 0.1025 | 0.0455 | 1.0000 | 0.6547 | - | 0.5637 | |||
| HTN | ||||||||||||
| Difference (%) | 2.9 | 8.3 | 0.0 | 5.7 | 10.5 | 2.9 | 0.0 | 5.9 | −2.9 | |||
| p value | 0.1797 | - | 1.0000 | - | - | - | 1.0000 | - | 0.3173 | |||
Values are presented as number (%). Group 1: 10–12 years old; Group 2: 13–18 years old.
AD = auscultatory device (Greenlight 300TM); EBP = elevated blood pressure; HTN = hypertension; MS = mercury sphygmomanometer; OD = oscillometric device (Omron HEM-907).
The percent agreement between MS and AD was 95.2%, which was higher than that between MS and OD (89.4%) (Supplementary Table 1). Within the same age and sex groups, the percent agreement between MS and AD was superior to that between MS and OD. Across all age and sex groups, the percent agreement between MS and AD was strong, exceeding 90%. The values were lower among girls (98.1% for boys and 92.2% for girls) and younger age groups (91.7% for Group 1 and 97.1% for Group 2). Among boys, agreement increased with age (94.7% for Group 1 and 100.0% for Group 2), and the same trend was observed among girls. The percent agreement between MS and OD was particularly low in the younger age groups of boys (78.9%) and girls (88.2%) in Group 1 (Supplementary Table 1).
The kappa index, which considers chance agreement, revealed moderate agreement between MS and AD, whereas the agreement between MS and OD was weak (0.77 for MS-AD, 0.47 for MS-OD). The kappa values between MS and AD were higher among boys than girls and in Group 2 than in Group 1. The kappa indices between MS and OD generally showed lower values than AD across age and sex groups. Among the kappa values between MS and OD, Group 1, the younger group, had a particularly low value of 0.21 (Supplementary Table 1).
DISCUSSION
In this study, we performed BP measurements concurrently in children and adolescents in the KNHANES to evaluate the BP differences between MS and AD (Greenlight 300TM) or between MS and OD (Omron HEM-907), which could replace MS in future surveys. Through this study, we identified the following points: the MS group had the highest BP values, followed by the AD and OD groups. There were no significant differences in the SBP or DBP between the MS and AD groups. In the case of MS and OD, D-SBP was insignificant, whereas D-DBP was substantial. Regarding the correlation between devices, MS and AD showed a good correlation, while MS and OD were relatively low, especially DBP, which was the lowest in girls in Group 1, and girls and boys in Group 2, showing a moderate correlation. Similarly, the absolute error was tolerable for AD and SBP of OD, but not for DBP of OD, according to the AAMI standards. In multivariate analysis, AC was negatively correlated with BP differences in both AD and OD, except for D-SBP in OD. The prevalence of hypertension measured by AD was the same as that measured by MS; however, the prevalence measured by OD decreased. When BP was measured using OD, hypertension and EBP + HTN were underestimated, especially in Group 1.
In this study, MS correlated better with AD, and OD differed remarkably from MS in DBP. In particular, OD did not meet the AAMI standards and recently published A Universal Standards for Validation of the Blood Pressure Measuring Device in 2018 (criterion 1); a tolerable error of 10 mmHg or less and an estimated probability of that error of at least 85% is acceptable.15) Therefore, AD seems more suitable as a device to replace MS. Similar results were announced in the studies comparing BP devices conducted on adults in Korea.12),13) In a study in which Choi et al.12) compared MS vs. AD and MS vs. OD, respectively, there was no significant difference in BP between MS and AD (MS-AD, SBP −0.52±4.12 mmHg, DBP −0.78±3.23 mmHg) with high correlation. But, differences in DBP were measured between MS and OD (MS-OD, SBP −0.62±5.62 mmHg, DBP 6.23±5.62 mmHg) with significant differences in hypertension prevalence (MS vs. OD, 12.5% vs. 11.2%, p=0.03). A comparative study of devices based on data from the National Health and Nutrition Examination Survey (NHANES) in the United States showed a difference in DBP between MS and OD, similar to the results of our study. Ostchega et al.23) compared MS and OD (Omron HEM-907XL) in subjects aged 13 years and older and showed good correlation and satisfied the AAMI standards except for DBP measured in participants aged 13–19 years (MS-OD, 1.77±8.65 mmHg). However, in the comparative study with MS and AD, SBP difference was contrasted from the results of our study in subjects between the ages of 8 and 17 years (MS-AD, −1.10±4.87 mmHg).24) Using a different device in this study (Welch Allyn 767) may have contributed to the difference from our results. Nonetheless, there was no significant difference in the prevalence of hypertension.
Studies comparing BP devices in the pediatric age group often have relatively small sample sizes and are rarely well-designed, resulting in mixed results. In a meta-analysis of 29 articles with 26,879 children, the pooled studies varied widely depending on the instrument manufacturer, study setting, and recorder.25) OD showed higher SBP values than MS (pooled effect estimate of MS-OD −2.53 mmHg; 95% confidence interval [CI], 0.57–4.50). The difference was exceptionally high when the Dinamap 8100 model devices were used, high for school-based studies, and reduced when the validated devices were used. There was no significant difference for DBP (pooled effect estimate of MS-OD −1.55 mmHg; 95% CI, −0.2–3.31); however, there was heterogeneity between studies. Additionally, if DBP was defined as only K-4 or K-4 (or K-5) using MS, OD-measured DBP was significantly lower than MS measurements (pooled effect estimate of MS-OD, 5.74 mmHg; 95% CI, 2.98–8.51). In contrast, in our study, despite defining K-5 as DBP,8) it was lower in OD than in MS, which may be because the BP readers in our study were well-trained and measured BP more accurately. If DBP was defined as K-4, the difference in DBP between the MS and OD groups would have been greater. In another meta-analysis of 28 articles, ODs overestimated SBP in 1.17 mmHg on average compared to MS, but the difference in DBP was insignificant; however, there was also heterogeneity in the device model, study environment and observer training,26) implying that there are the limitations in reliability.
When analyzing the factors influencing the BP differences between MS and AD, both D-SBP and D-DBP had a negative correlation with AC, and MS measurement was also associated with D-DBP. This may be influenced by the difficulty in checking pulses in children with small AC, which makes auscultation difficult to measure BP. When analyzed by arm quartiles, we found that the BP difference between the MS and AD groups increased as AC decreased. Similarly, it was found that AC was negatively correlated with BP differences between MS and AD in adult studies.12),13) Conversely, no factors were associated with MS and OD in D-SBP, but D-DBP was related to MS and AC, similar to adult studies.12),13) The thinner the arm, the more difficult it was to detect the vibration of the blood vessels in the ODs, which is especially noticeable for DBP. Even when viewed by quartile for AC, there was a significant difference from Q1 to Q3; the smallest difference was observed in Q4. Other factors such as age and sex do not appear to be meaningful, indicating that the accuracy of BP measurement in children seems to be highly related to AC; therefore, it is crucial to accurately measure the AC and select the appropriate BP cuff when measuring BP in the future, and further studies with larger sample size are needed. However, caution should be exercised when interpreting the statistical significance of this pediatric study due to multiple comparisons.
Since the prevalence of hypertension is lower in children than in adults, and because of the small number of participants in this study, it was difficult to analyze the difference in the frequency of hypertension according to the device, and the analysis of subgroups by age and sex was unreliable. In addition, since the definition of hypertension differs according to the guidelines in children, it is possible that the frequency of hypertension would have been different if hypertension had been defined according to other guidelines that used the same criteria as adults, such as those aged 13 years and older or 16 years and older.8),9) However, in the frequency of EBP + HTN, MS and AD had a high concordance rate compared to MS and OD, confirming the superiority of AD. The overall prevalence difference between MS and OD was more significant than that between MS and AD, particularly for EBP + HTN in Group 1 (p=0.0143). In a study of adults in Korea, there was no difference in the frequency of hypertension between MS and AD, but there was a significant difference between MS and OD (MS vs. AD,10.3% vs. 10.5%; MS vs. OD, 10.4% vs. 9.4%).12) On the other hand, a study conducted on 4,689 people over the age of 18 years based on the NHANES in the United States reported that there was no difference in the frequency of hypertension or stage 2 hypertension in BP measured by HEM-908XL and mercury BP monitors, indicating that there are differences between devices.27)
Our study had some limitations. First, AD showed superiority over OD but did not compare all ODs, and Omron HEM-907 did not represent OD. In addition, although the number of subjects was determined based on the AAMI criteria, the number of subjects was small, and a more extensive study is required. Third, the definition of hypertension in children is BP measured over 3 visits, but in this study, it was defined as 3 measurements at one visit, which may differ from actual hypertension. Nevertheless, it is significant that this study measured the 3 types of devices at once and compared them simultaneously and obtained relatively reliable results by randomizing the measurement order in a well-organized setting.
In conclusion, MS and AD had no significant difference for BP values and a good correlation. The difference in SBP was not significant; however, DBP was lower in OD and showed a moderate correlation. The absolute error distribution was also lower than the standards for DBP in OD. BP difference was negatively correlated with AC, except for D-SBP in OD. The prevalence of hypertension was similar in MS and AD but underestimated in OD. The superiority of AD over OD in children and adolescents suggests that AD is a possible alternative in the National Survey.
Footnotes
Funding: This study was partly supported by a grant from the Wonkwang University 2022.
Conflict of Interest: The authors have no financial conflicts of interest.
Data Sharing Statement: The data generated in this study is available from the corresponding author upon reasonable request.
- Conceptualization: Kim SH, Shin J, Lee EM.
- Formal analysis: Kim YM.
- Methodology: Kim SH, Shin J, Lee EM.
- Supervision: Lee EM.
- Writing - original draft: Kim SH, Kim YM, Lee EM.
- Writing - review & editing: Kim SH, Kim YM, Lee EM.
SUPPLEMENTARY MATERIALS
Percent agreements and kappa indices for the prevalence of elevated blood pressure and hypertension according to sex and age
The scatter plots and correlations between MS and AD, as well as between MS and OD for SBP and DBP according to sex and age groups. Group 1: 10–12 years old; Group 2: 13–18 years old.
References
- 1.Flynn J. The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr Nephrol. 2013;28:1059–1066. doi: 10.1007/s00467-012-2344-0. [DOI] [PubMed] [Google Scholar]
- 2.Song P, Zhang Y, Yu J, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173:1154–1163. doi: 10.1001/jamapediatrics.2019.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos LE, Oren A, Uiterwaal C, Gorissen WH, Grobbee DE, Bots ML. Adolescent blood pressure and blood pressure tracking into young adulthood are related to subclinical atherosclerosis: the Atherosclerosis Risk in Young Adults (ARYA) study. Am J Hypertens. 2003;16:549–555. doi: 10.1016/s0895-7061(03)00857-4. [DOI] [PubMed] [Google Scholar]
- 4.Juhola J, Magnussen CG, Viikari JS, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–590. doi: 10.1016/j.jpeds.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108–1115. doi: 10.1161/HYPERTENSIONAHA.115.05831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DH, Cho IJ, Kim W, et al. Elevated on-treatment diastolic blood pressure and cardiovascular outcomes in the presence of achieved systolic blood pressure targets. Korean Circ J. 2022;52:460–474. doi: 10.4070/kcj.2021.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho JY, Cho DH, Youn JC, et al. Korean Society of Heart Failure guidelines for the management of heart failure: definition and diagnosis. Korean Circ J. 2023;53:195–216. doi: 10.4070/kcj.2023.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 9.Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887–1920. doi: 10.1097/HJH.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 10.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 11.Minamata Convention on Mercury - text and annexes. UN environment 362 programme [Internet] Chatelaine: Minamata Convention on Mercury; 2013. [cited 2015 March 15]. Available from: https://minamataconvention.org/en/documents/minamata-convention-mercury-text-and-annexes. [Google Scholar]
- 12.Choi S, Kim YM, Shin J, et al. Comparison of the accuracy and errors of blood pressure measured by 2 types of non-mercury sphygmomanometers in an epidemiological survey. Medicine (Baltimore) 2018;97:e10851. doi: 10.1097/MD.0000000000010851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YM, Ohn DW, Kim SH, et al. Direct comparison of an automated oscillometric device with an electronic auscultatory device for epidemiologic survey to evaluate the prevalence of hypertension. Medicine (Baltimore) 2022;101:e32299. doi: 10.1097/MD.0000000000032299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Association for the Advancement of Medical Instrumentation (AAMI) American National Standards for Electronic or Automated Sphygmomanometers. Arlington (VA): AAMI; 2002. [Google Scholar]
- 15.Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) collaboration statement. J Hypertens. 2018;36:472–478. doi: 10.1097/HJH.0000000000001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graves JW, Tibor M, Murtagh B, Klein L, Sheps SG. The Accoson Greenlight 300, the first non-automated mercury-free blood pressure measurement device to pass the International Protocol for blood pressure measuring devices in adults. Blood Press Monit. 2004;9:13–17. doi: 10.1097/00126097-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 17.El Assaad MA, Topouchian JA, Darné BM, Asmar RG. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241. doi: 10.1097/00126097-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien E, Petrie J, Littler W, et al. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11:677–679. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Lee EM, Kim Y, Kim SH, Sung K, Ihm S, Shin J. Quality Control and Assurance of Blood Pressure Measurement: Korea National Health and Nutrition Examination Survey 2019. Sejong: Korea Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 20.Kim HL, Park SM, Cho IJ, et al. Standardized protocol of blood pressure measurement and quality control program for the Korea National Health and Nutrition Examination Survey. Clin Hypertens. 2023;29:28. doi: 10.1186/s40885-023-00252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American National Standards Institute (ANSI) Non-invasive sphygmomanometers - part 2: clinical investigation of automated measurement type. ANSI/AAMI/ISO 81060-2. New York (NY): ANSI; 2013. [cited 2017 July]. Available from: http://webstore.ansi.org. [Google Scholar]
- 22.Kim SH, Park Y, Song YH, et al. Blood pressure reference values for normal weight Korean children and adolescents: data from The Korea National Health and Nutrition Examination Survey 1998-2016: The Korean Working Group of Pediatric Hypertension. Korean Circ J. 2019;49:1167–1180. doi: 10.4070/kcj.2019.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostchega Y, Nwankwo T, Sorlie PD, Wolz M, Zipf G. Assessing the validity of the Omron HEM-907XL oscillometric blood pressure measurement device in a National Survey environment. J Clin Hypertens (Greenwich) 2010;12:22–28. doi: 10.1111/j.1751-7176.2009.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostchega Y, Prineas RJ, Nwankwo T, Zipf G. Assessing blood pressure accuracy of an aneroid sphygmomanometer in a national survey environment. Am J Hypertens. 2011;24:322–327. doi: 10.1038/ajh.2010.232. [DOI] [PubMed] [Google Scholar]
- 25.Duncombe SL, Voss C, Harris KC. Oscillometric and auscultatory blood pressure measurement methods in children: a systematic review and meta-analysis. J Hypertens. 2017;35:213–224. doi: 10.1097/HJH.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 26.Araujo-Moura K, Souza LG, Mello GL, De Moraes AC. Blood pressure measurement in pediatric population: comparison between automated oscillometric devices and mercury sphygmomanometers-a systematic review and meta-analysis. Eur J Pediatr. 2022;181:9–22. doi: 10.1007/s00431-021-04171-3. [DOI] [PubMed] [Google Scholar]
- 27.Ostchega Y, Hughes JP, Kit B, et al. Differences in Hypertension and stage ii hypertension by demographic and risk factors, obtained by two different protocols in US adults: National Health and Nutrition Examination Survey, 2017-2018. Am J Hypertens. 2022;35:619–626. doi: 10.1093/ajh/hpac042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent agreements and kappa indices for the prevalence of elevated blood pressure and hypertension according to sex and age
The scatter plots and correlations between MS and AD, as well as between MS and OD for SBP and DBP according to sex and age groups. Group 1: 10–12 years old; Group 2: 13–18 years old.