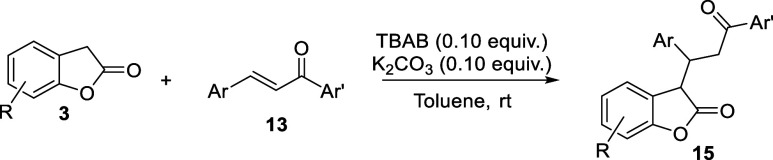
Table 4. Analysis of the Scope with 2-Coumaranones 3 and Chalcones 13.
| entry | lactones | chalcones | Ar | Ar′ | time (h) | yield (%)a | d.rb |
|---|---|---|---|---|---|---|---|
| 1 | 3a, R = H | 13a | Ph | Ph | 4 | 15a, 81 | 64/36 |
| 2 | 3a, H | 13b | 4-ClC6H4 | Ph | 5.5 | 15b, 83 | 53/47 |
| 3 | 3a, H | 13f | 4-MeOC6H4 | Ph | 5 | 15c, 87 | 54/46 |
| 4 | 3b, 5-OAc | 13a | Ph | Ph | 2.5 | 15d, 89 | 59/41 |
| 5 | 3a, H | 13i | Ph | 4-MeOC6H4 | 5 | 15e, 83 | 62/38 |
| 6 | 3a, H | 13d | 2-ClC6H4 | Ph | 8.5 | 15f, 81 | 54/46 |
| 7 | 3a, H | 13e | 4-FC6H4 | Ph | 6.5 | 15g, 79 | 64/36 |
| 8c | 3c, 5-Cl | 13a | Ph | Ph | 36 | 15h, 75 | 63/37 |
| 9d | 3d, 5-NO2 | 13a | Ph | Ph | 24 | -e | - |
| 10d | 3d, 5-NO2 | 13f | 4-MeOC6H4 | Ph | 40 | -e | - |
Isolated yield.
Determined by 1H NMR on the crude material.
Reaction temperature at 50 °C.
Reaction temperature at 80 °C.
Starting materials recovered.