Abstract
This study aims to identify risk factors for secondary venous thromboembolism (VTE) in stroke patients and establish a nomogram, an accurate predictor of probability of VTE occurrence during hospitalization in stroke patients. Medical Information Mart for Intensive Care IV (MIMIC-IV) database of critical care medicine was utilized to retrieve information of stroke patients admitted to the hospital between 2008 and 2019. Patients were randomly allocated into train set and test set at 7:3. Univariate and multivariate logistic regression analyses were used to identify independent risk factors for secondary VTE in stroke patients. A predictive nomogram model was constructed, and the predictive ability of the nomogram was evaluated using receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA). This study included 266 stroke patients, with 26 patients suffering secondary VTE after stroke. A nomogram for predicting risk of secondary VTE in stroke patients was built according to pulmonary infection, partial thromboplastin time (PTT), log-formed D-dimer, and mean corpuscular hemoglobin (MCH). Area under the curve (AUC) of the predictive model nomogram was 0.880 and 0.878 in the train and test sets, respectively. The calibration curve was near the diagonal, and DCA curve presented positive net benefit. This indicates the model's good predictive performance and clinical utility. The nomogram effectively predicts the risk probability of secondary VTE in stroke patients, aiding clinicians in early identification and personalized treatment of stroke patients at risk of developing secondary VTE.
Keywords: MIMIC-IV, stroke, venous thromboembolism, nomogram
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is one of the top 5 most common vascular diseases in most countries, affecting as many as 5% of the population.1–3 An estimated 1 220 000 cases of VTE occur annually in the U.S. according to a 2021 report from the American Heart Association. 4 The lifetime risk of VTE in adults (≥45 years old) in the U.S. is 8%. 5 A nationwide population-based cohort study lasting 30 years showed that approximately 20% of individuals die within 1 year after being diagnosed with VTE, usually due to disease events that lead to the occurrence of VTE, including stroke. 6
VTE is a complication of stroke and ranks as the third leading cause of post-stroke mortality following the stroke itself and associated infections. 7 The subsequent risk increases during the acute phase of stroke and within the initial three months post-event, 8 with a twofold increase in mortality risk for affected patients. 9 Additionally, the occurrence of stroke-related VTE is associated with lower short-term and long-term survival rates, increased disability, and prolonged hospital stays. 10 Thus, lowering the death and disability rate of stroke patients greatly depends on discovering predicting indicators for secondary VTE in stroke, early detection, and prevention. Zhu et al suggested using the Caprini score to assess risk of secondary VTE in stroke patients. 11 Nonetheless, the Caprini score is based on static assessment and cannot dynamically reflect changes in patient condition. 12
A dynamic visualization tool called nomogram can be employed to evaluate and determine the exact risk of specific patient outcomes or clinical events. In order to create a novel nomogram model for predicting the likelihood of secondary VTE in patients, this study integrated the laboratory tests, demographic data, and complications of stroke patients. This allows for individualized prevention and therapy.
Methods
Data Source
Medical Information Mart for Intensive Care (MIMIC) is a critical care medicine database established by Beth Israel Deaconess Medical Center (BIDMC) at Harvard Medical School, MIT Laboratory for Computational Physiology, Oxford University, and emergency physicians, intensivists, and computer science experts from Massachusetts General Hospital. MIMIC contained data from all patients at BIDMC, and the team de-identified patient information to protect privacy and made it freely available to investigators worldwide. The most recent version, MIMIC-IV, gathered clinical data from 450 000 hospital admissions and more than 190 000 patients at BIDMC between 2008 and 2019. It contained detailed information about vital signs, surgical procedures, laboratory test results, comorbidities, medication records, demographics, and follow-up survival status. For clinical decision-making and medical research, it is extremely important.
Study Population
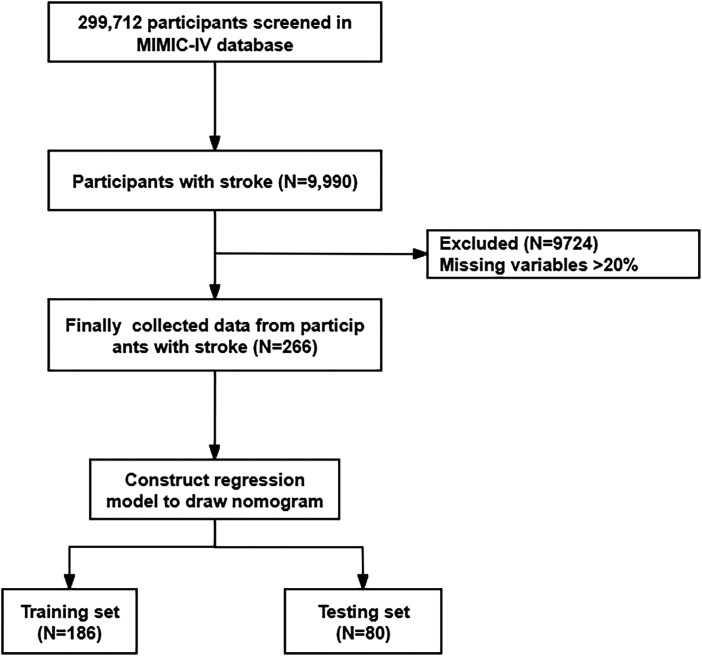
This study was a retrospective analysis based on the MIMIC-IV database. The database did not contain any sensitive information and did not require ethical approval. Between 2008 and 2019, the MIMIC-IV database included 299 712 participants, including 9990 stroke patients. Stroke was defined as 4 subtypes, namely Acute Ischemic Stroke (AIS), Intracerebral Hemorrhage (ICH), Subarachnoid Hemorrhage (SAH), and Transient Ischemic Attack (TIA). The diagnostic codes were as follows 13 : AIS: 362.3, 433.x1, 434.x1, 436 (ICD 9); H34.1, I63.x, I64.x (ICD 10); ICH: 431.x (ICD 9); I61.x (ICD 10); SAH: 430.x (ICD 9); I60.x (ICD 10); TIA: 435.x (ICD 9); G45.x (ICD 10). Subsequently, the study excluded 9724 patients with missing variables >20%. 266 stroke patients were finally included and randomly assigned to a train set (N = 186) and a test set (N = 80) in a 7:3 ratio. The detailed screening process is shown in Figure 1.
Figure 1.
Flowchart of patient screening process.
Variable Collection
Variable collection could be divided into 3 categories: (1) Demographics, including gender, age, race, and marital status. (2) Complications, including VTE 14 (deep vein thrombosis (DVT) and pulmonary embolism (PE)), encoded using ICD codes. DVT: 452, 453 (ICD 9); I801, I828, I829, O223, I822, I820, I802, I81, O082, I823, O873 (ICD 10); PE: 415, 673 (ICD 9); O882, I269, I260 (ICD 10), lung infection, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), kidney disease, liver disease. (3) Biochemical indicators (collected within 24 h of admission), including D-dimer, anion gap, bicarbonate concentration, chloride ion concentration, hematocrit, hemoglobin, sodium ion concentration, potassium ion concentration, mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), platelet count, red blood cell distribution width (RDW), white blood cell count (WBC), partial thromboplastin time (PTT), international normalized ratio (INR), prothrombin time (PT), creatinine level, blood glucose. Note: The value corresponding to the most severe degree was selected for indicators with multiple measurement results.
Statistical Analysis
Data retrieval from the MIMIC-IV (version 2.2) database was performed using Structured Query Language (SQL), and data analysis was conducted using the R (version 4.2.3) software. Samples with missing variable proportions exceeding 20% were excluded, and other missing variables were handled using the Random Forest (RF) method in mice package. The tableone package was utilized to construct the baseline table, with continuous variables represented as median (IQR), and between-group comparisons done using Wilcoxon–Mann–Whitney test. The categorical data was represented as a percentage (%) and compared using the chi-square test. A two-sided P-value < 0.05 was deemed statistically significant. Before constructing the nomogram, the samples were randomly assigned to the train and test sets in a 7:3 ratio. The glm package was utilized to construct a univariate logistic regression model to identify potential factors leading to adverse outcomes in the train set. The variables obtained from LASSO regression were analyzed using multivariate logistic regression analysis. According to the Akaike information criterion (AIC), a two-sided stepwise regression was performed in the multivariate logistic regression model to determine independent predictive factors, where variables with P < 0.1 were included in the final model. The regplot package was utilized to plot nomogram and output risk scores of predictive factors. The rms package was utilized to predict risk probabilities of samples. Model performance was evaluated using C-index and AUC calculated by Hmisc and pROC packages, respectively. The predictive performance of the nomogram was evaluated using the calibration curve with 1000 resampling and the Hosmer–Lemeshow goodness-of-fit test. The rmda package was utilized to plot decision curve analyses (DCA) to assess the clinical value of the nomogram.
Results
Baseline Characteristics
From 2008 to 2019, 266 stroke patients met the criteria in MIMIC-IV. Based on whether stroke patients had secondary VTE, they were divided into the VTE group (N = 26) and the non-VTE group (N = 240), and baseline characteristics of patients are listed in Table 1. Significant variances were seen in WBC, INR, PT, D-dimer, and pulmonary infection (P-value < 0.05). Compared to non-VTE group, VTE group had high WBC (11.15 vs 8.3 P = 0.015), INR (1.3 vs 1.1, P = 0.002), PT (14.2 vs 12.55, P = 0.002), D-dimer (4180.0 vs 1093.5, P < 0.001) and a high proportion of lung infections (57.7% vs 9.2%, P < 0.001).
Table 1.
Participants Characteristics of Included Patients Grouped by VTE.
| Characteristics | Total | Non-VTE | VTE | P-Value |
|---|---|---|---|---|
| (N = 266) | (N = 240) | (N = 26) | ||
| Age (years) | 67.00 [54.00, 76.00] | 67.00 [54.00, 77.00] | 61.00 [54.25, 74.75] | 0.446 |
| Gender | 0.713 | |||
| Female | 129 (48.5) | 115 (47.9) | 14 (53.8) | |
| Male | 137 (51.5) | 125 (52.1) | 12 (46.2) | |
| Race | 0.222 | |||
| White | 163 (61.3) | 143 (59.6) | 20 (76.9) | |
| Black | 39 (14.7) | 37 (15.4) | 2 (7.7) | |
| Other | 64 (24.1) | 60 (25.0) | 4 (15.4) | |
| Marital status | 0.812 | |||
| Married | 122 (45.9) | 109 (45.4) | 13 (50.0) | |
| Unmarried | 144 (54.1) | 131 (54.6) | 13 (50.0) | |
| Anion gap (mmol/L) | 15.00 [13.00, 17.00] | 14.00 [13.00, 17.00] | 15.00 [14.00, 17.75] | 0.186 |
| Bicarbonate (mmol/L) | 24.00 [21.00, 26.00] | 24.00 [21.00, 26.00] | 23.00 [20.00, 25.00] | 0.274 |
| Chloride (mmol/L) | 104.00 [101.00, 106.75] | 104.00 [101.00, 106.25] | 104.50 [102.00, 106.75] | 0.816 |
| Hematocrit | 35.80 [29.80, 39.95] | 35.95 [29.87, 40.10] | 33.50 [29.22, 37.95] | 0.200 |
| Hemoglobin (g/dL) | 11.90 [9.60, 13.20] | 12.00 [9.67, 13.20] | 10.60 [9.33, 13.17] | 0.377 |
| Sodium (mEq/L) | 140.00 [137.00, 141.00] | 140.00 [137.00, 142.00] | 139.00 [137.00, 140.00] | 0.277 |
| Potassium (K/µL) | 4.10 [3.80, 4.50] | 4.10 [3.80, 4.50] | 4.00 [3.82, 4.80] | 0.962 |
| MCH (pg) | 29.90 [28.60, 31.30] | 29.90 [28.50, 31.20] | 30.20 [29.45, 31.70] | 0.175 |
| MCHC (g/L) | 32.80 [31.92, 33.70] | 32.80 [31.90, 33.70] | 32.75 [32.00, 34.98] | 0.483 |
| MCV (fL) | 90.00 [87.00, 94.00] | 90.00 [87.00, 94.00] | 90.50 [86.25, 94.75] | 0.856 |
| Platelet (K/µL) | 182.00 [135.25, 241.00] | 183.50 [138.00, 239.00] | 161.50 [113.00, 236.00] | 0.456 |
| RDW | 14.00 [13.10, 15.90] | 13.95 [13.07, 15.90] | 14.40 [13.57, 15.70] | 0.261 |
| WBC (K/µL) | 8.40 [6.50, 11.57] | 8.30 [6.40, 11.30] | 11.15 [8.10, 16.53] | 0.015 |
| INR | 1.20 [1.10, 1.30] | 1.10 [1.10, 1.30] | 1.30 [1.20, 1.40] | 0.002 |
| PT (s) | 12.70 [11.70, 14.70] | 12.55 [11.60, 14.40] | 14.20 [12.72, 16.00] | 0.002 |
| PTT (s) | 30.10 [27.52, 36.65] | 29.85 [27.50, 36.50] | 34.05 [28.02, 48.30] | 0.176 |
| Creatinine (μmol/L) | 0.90 [0.70, 1.20] | 0.90 [0.70, 1.20] | 1.00 [0.80, 1.37] | 0.304 |
| Glucose (mg/dL) | 109.00 [93.00, 138.75] | 109.00 [93.00, 136.38] | 119.17 [93.00, 154.12] | 0.382 |
| D-dimer (ng/mL FEU) | 1193.00 [555.00, 2978.00] | 1093.50 [491.25, 2593.50] | 4180.00 [1808.25, 7236.00] | <0.001 |
| Lung infection | <0.001 | |||
| No | 229 (86.1) | 218 (90.8) | 11 (42.3) | |
| Yes | 37 (13.9) | 22 (9.2) | 15 (57.7) | |
| Congestive heart failure | 0.324 | |||
| No | 227 (85.3) | 207 (86.2) | 20 (76.9) | |
| Yes | 39 (14.7) | 33 (13.8) | 6 (23.1) | |
| Chronic pulmonary disease | 1.000 | |||
| No | 220 (82.7) | 198 (82.5) | 22 (84.6) | |
| Yes | 46 (17.3) | 42 (17.5) | 4 (15.4) | |
| Liver disease | 0.406 | |||
| No | 242 (91.0) | 220 (91.7) | 22 (84.6) | |
| Yes | 24 (9.0) | 20 (8.3) | 4 (15.4) | |
| Renal disease | 0.427 | |||
| No | 215 (80.8) | 196 (81.7) | 19 (73.1) | |
| Yes | 51 (19.2) | 44 (18.3) | 7 (26.9) |
WBC, white blood cell; RDW, red cell distribution width; PTT, partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; BUN, blood urea nitrogen; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; VTE, venous thromboembolism.
To reduce bias and confounding factors, the total sample was randomly assigned into the train set (N = 186) and test set (N = 80) in a 7:3 ratio, as presented in S1 Table. Except for creatinine, anion gap, and bicarbonate concentration, which had intergroup differences, there were no significant variances in other variables (P > 0.05).
Investigation of Factors for VTE Development in Stroke Patients
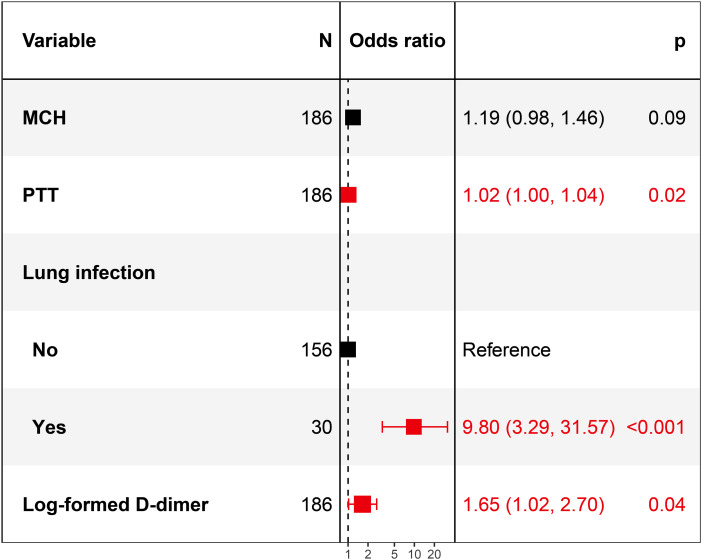
Multivariate logistic model (Figure 2) found PTT (OR = 1.02, 95CI%: 1.00-1.04, P = 0.02), lung infection (OR = 9.8, 95CI%: 3.29-31.57, P < 0.001) and log-formed D-dimer (OR = 1.65, 95CI%: 1.02-2.7, P = 0.04) were independent risk factors for secondary VTE in stroke patients.
Figure 2.
Forest plot of multivariate logistic regression model.
Risk Prediction Nomogram and Verification
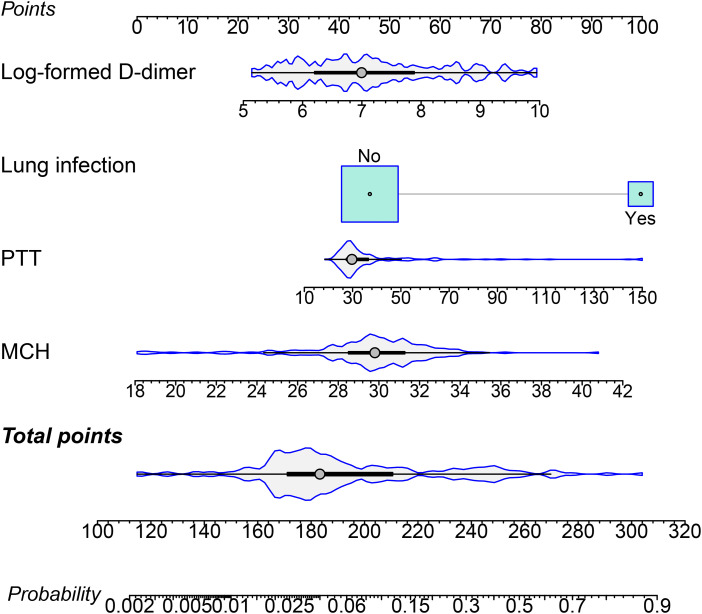
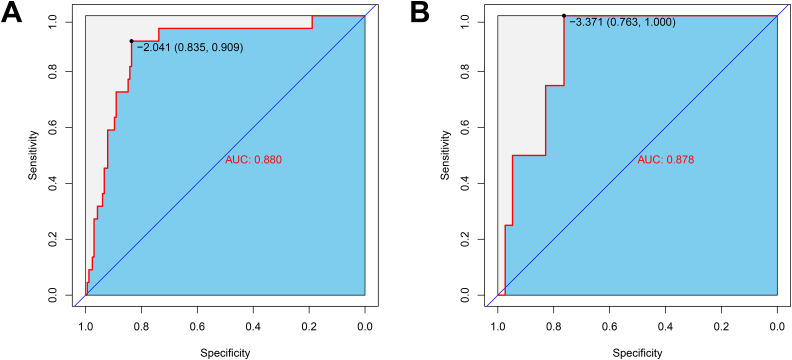
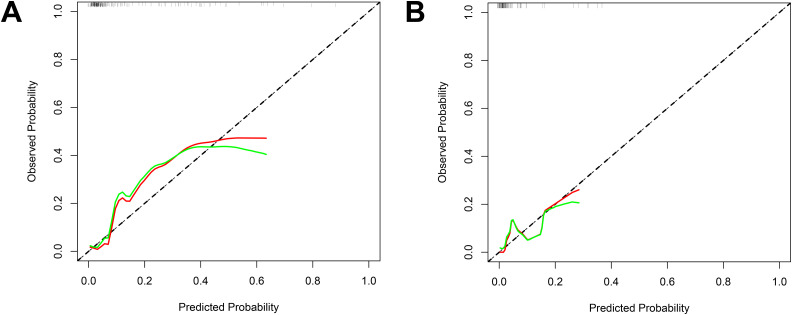
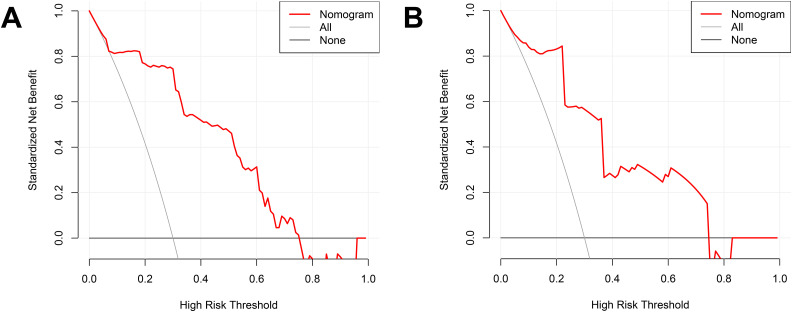
By integrating risk factors obtained in the previous chapter, a nomogram was built to predict the probability of VTE in stroke patients during hospitalization (Figure 3). The AUC of the nomogram in the train set was 0.880 (Figure 4A), and AUC in test set was 0.878 (Figure 4B). The C index of he nomogram was 0.854. P values of the Hosmer–Lemeshow test in the train set and test set were 0.400 and 0.964, respectively, indicating a high goodness-of-fit of the model. Calibration curve of the nomogram was illustrated in Figure 5, which was obtained by resampling 1000 times. The diagonal line was the reference line. The calibration curve was close to the diagonal line, indicating good consistency between nomogram in the train set and test set and actual prognosis results. DCA curves of the train set and test set are plotted in Figure 6. The horizontal axis represented no sample intervention, and the net profit was 0. The diagonal line represented that everyone had accepted intervention. Within the threshold probability range, significant positive net profit was obtained after intervention, indicating that the nomogram had good decision-making ability.
Figure 3.
Nomogram for predicting probability of VTE in participants.
The size of boxes (cyan) represents the difference of the relative proportion of patients in each subgroup. The density plot (gray) of total points shows its distribution.
Figure 4.
The ROC curve of the nomogram.
(A) Train set; (B) test set. The variables entered in the nomogram are the same.
Figure 5.
Calibration curves of the nomograms.
(A) Train set; (B) test set. The diagonal represents a perfect prediction by an idea model. The red line and green line represent the initial cohort and bias corrected by bootstrapping (B = 1000 repetitions), respectively.
Figure 6.
DCA curves of the nomogram.
(A) Train set; (B) test set. The horizontal line indicates no participants develop VTE, and the gray oblique line indicates participants develop VTE. The red solid line represents the VTE risk nomogram.
Discussion
We focused on stroke patients based on MIMIC-IV, a large public database, and found that 9.8% of patients developed VTE during hospitalization. As age increases, the patient's blood circulation gradually deteriorates, and reduced activity levels elevates risk of VTE. 15 In this work, patient population consisted of stroke patients in the hospital, and factors such as long-term bed and limitation of activity make critically ill patients more prone to VTE complications. 16 Thus, for stroke patients who are severely ill, early prevention of VTE incidence is essential.
D-dimer is a product of fibrin degradation, reflecting ongoing coagulation and fibrinolysis processes.17,18 An elevated risk of VTE is linked to elevated D-dimer levels.19,20 D-dimer is a biomarker that shows promise in determining a stroke patient's likelihood of developing secondary VTE.19,21,22 Furthermore, a study stratifying patients with different types of solid tumors according to VTE risk found that certain types of solid tumors (esophageal, lung, melanoma, ovarian, pancreatic, gastric, and uterine) were associated with a higher incidence of VTE compared to other cancer types (6.8% vs 3.9%, p < 0.001). Additionally, multivariate analysis indicated an increased incidence of ischemic stroke in the high-risk VTE group (OR = 1.22, p < 0.001). 23 These findings demonstrate an increased risk of VTE and arterial thrombosis associated with different cancer types, with a positive correlation observed between VTE risk and arterial thrombosis formation, which becomes more significant with disease severity. 23 However, Li et al's study did not find a role for D-dimer in secondary VTE following stroke. 24 To reduce data dispersion, we performed a logarithmic transformation on D-dimer to better fit the data for linear modeling and correlation analysis. The results showed that log-transformed D-dimer is an independent risk factor for secondary VTE in stroke patients.
Coagulation screening assays like PTT and activated PTT are often utilized in laboratory.25,26 Activated PTT is presently more widely utilized in clinical practice because, in comparison to PTT, it comprises specific activators that allow for more precise determination of activity of certain coagulation components. 27 Prolonged activated PTT is a risk feature for thrombosis.28,29 Mao et al found that critically ill COVID-19 patients typically exhibit coagulation dysfunction, characterized by elevated D-dimer levels and prolonged prothrombin time (PT), primarily as a result of endothelial dysfunction caused by inflammatory response. 30 Critically ill patients, due to severe pulmonary infection, experience hypoxia, leading to inflammation and consequent endothelial injury and slowed blood flow. Moreover, activated partial thromboplastin time (PTT) is significantly higher in severe cases compared to non-severe cases, indicating increased coagulability in these patients, which raises the risk of stroke. 31 A meta-analysis incorporating 77 randomized controlled trials (38 732 cases) revealed stroke incidence rates among COVID-19 patients of 0.168% in the overall population and 0.175% in hospitalized populations. 32 These rates are lower than previously reported levels, which ranged from 0.9% to 5%,33–36 likely due to earlier studies being limited to the first wave of the pandemic. However, in studies of critically ill COVID-19 patients admitted to intensive care units, 2.2% experienced acute stroke: 0.7% ischemic stroke and 1.0% hemorrhagic stroke, which correlated with the presence of traditional vascular risk factors. 37 Violi et al reported that during the acute phase of pneumonia, a hypercoagulable state may precipitate arterial and/or venous thrombosis. 38 A large healthcare system in New York City reported a thrombotic event rate of 16.0% among hospitalized COVID-19 patients, with D-dimer levels at admission independently associated with thrombotic events, consistent with early coagulation dysfunction. 39 Therefore, future attention is warranted on coagulation function in stroke patients, particularly D-dimer levels and prolonged activated PTT, and their impact on the risk of secondary VTE in stroke patients. As the MIMIC database did not provide data on activated PTT, we only analyzed PTT and found that prolonged PTT was independently associated with secondary VTE in stroke patients, which is also a limitation of our study design.
Around 10% of stroke patients develop pulmonary infections, such as pneumonia.40–42 Inflammatory response triggered by pulmonary infections may activate coagulation system, thus facilitating thrombus formation.40,43,44 Clinical data presented that pulmonary infections are associated with an increased risk of secondary VTE in stroke patients,24,45 and may also lead to death in stroke patients.46,47 Although the gold standard of stroke guidelines is to initiate treatment immediately after diagnosing an infection, multiple studies have reported that preventive treatment is more effective.48,49 But Vermeij JD et al's study presented different results, as preventive antibiotic treatment does not improve functional outcomes or mortality rates in stroke patients, although it reduces the risk of urinary tract infections in the population, but no preventive effect on pneumonia is found. 50 In fact, due to the difficulty in obtaining non-ventilated sputum specimens from stroke patients and the significant differences in antibiotic resistance rates in different regions of the world, there is currently no universal guideline to guide the management of pulmonary infections in stroke patients. When determining appropriate preventive antibiotic treatment, local resistance patterns need to be fully considered.
MCH is obtained by dividing total amount of hemoglobin by red blood cell count, used to quantify the amount of hemoglobin in each red blood cell, usually combined with other red blood cell indices to assess indicators of anemia. 51 Currently, there is limited research on the correlation between MCH and thrombosis. A case report suggests that when MCH and MCHC are significantly elevated, it is necessary to incubate the blood sample under 37°C conditions as it may indicate the presence of potential venous thrombosis. 52 According to this work, high MCH levels of stroke patients are associated with secondary VTE. Prospective investigations are necessary to validate this conclusion.
Early use of anticoagulants helps reduce incidence and mortality of thrombotic events in stroke patients.53,54 This also explains why identifying high-risk populations is important. Considering that the dynamic nomogram model can describe the probability of complications risk as factors (such as age, gender, biochemical indicators) change more accurately, early prevention and treatment can be carried out to avoid inadequate or excessive treatment. We successfully constructed a nomogram model using log-formed D-dimer, pulmonary infection, PTT, and MCH, and validated the model calibration, discriminative ability, and clinical utility through calibration, ROC, and DCA curves, fully demonstrating the model’s accuracy and practicality. This model may prove useful in forecasting the likelihood of secondary VTE in stroke patients and aids in early identification of high-risk patients and prompt intervention measures.
This study incorporated patient data spanning nearly 10 years, providing a certain degree of clinical representativeness. However, it also has limitations. Firstly, variables such as the severity of stroke upon admission, levels of C-reactive protein, 19 cancer history,23,55 mechanical ventilation, and markers of severity are common risk factors for secondary VTE in stroke patients, but we did not analyze them, which may have influenced the results. Secondly, the incidence of VTE in stroke patients is lower than in the general population, 15 which may lead to model overfitting and wider confidence intervals. Finally, our nomogram was constructed and validated based on the MIMIC-IV database, so external validation from different healthcare institutions is necessary.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296241254104 for Nomogram for Risk of Secondary Venous Thromboembolism in Stroke Patients: A Study Based on the MIMIC-IV Database by Folin Lan, Tianqing Liu, Celin Guan, Yufen Lin, Zhiqin Lin, Huawei Zhang, Xiaolong Qi, Xiaomei Chen and Junlong Huang in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Author Contribution: Conceptualization: Folin Lan, Junlong Huang
Data curation: Tianqing Liu, Yufen Lin
Formal Analysis: Folin Lan, Tianqing Liu
Investigation: Tianqing Liu, Huawei Zhang
Methodology: Folin Lan, Tianqing Liu, Xiaomei Chen
Project administration: Tianqing Liu, Yufen Lin
Resources: Celin Guan, Huawei Zhang
Software: Celin Guan, Xiaolong Qi
Supervision: Yufen Lin, Xiaolong Qi
Validation: Zhiqin Lin, Xiaomei Chen
Visualization: Zhiqin Lin, Xiaomei Chen
Writing – original draft: Folin Lan, Tianqing Liu, Junlong Huang
Writing – review & editing: Yufen Lin, Huawei Zhang, Celin Guan
Availability of Data and Materials: The data and materials in the current study are available from the corresponding author on reasonable request.
Consent for Publication: Not applicable.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: Before data from this study were included in the MIMIC-IV public database, all participants signed informed consent forms, adhered to the principles outlined in the Declaration of Helsinki, and were reviewed and approved by the NCHS Ethical Review Board.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was sponsored by Longyan City Science and Technology Plan Project Longyan City Science and Technology Plan Project, (grant number 2020LYF9012).
ORCID iD: Junlong Huang https://orcid.org/0009-0005-0295-9965
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. 2023;20(4):248-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffett L. Deep venous thrombosis. Ann Intern Med. 2022;175(9):ITC129-IITC44. [DOI] [PubMed] [Google Scholar]
- 3.Wendelboe AM, Raskob GE. Global burden of thrombosis: Epidemiologic aspects. Circ Res. 2016;118(9):1340-1347. [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: A report from the American heart association. Circulation. 2021;143(8):e254-e743. [DOI] [PubMed] [Google Scholar]
- 5.Bell EJ, Lutsey PL, Basu S, et al. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129(3):339. e19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sogaard KK, Schmidt M, Pedersen L, Horvath-Puho E, Sorensen HT. 30-year Mortality after venous thromboembolism: A population-based cohort study. Circulation. 2014;130(10):829-836. [DOI] [PubMed] [Google Scholar]
- 7.Field TS, Hill MD. Prevention of deep vein thrombosis and pulmonary embolism in patients with stroke. Clin Appl Thromb Hemost. 2012;18(1):5-19. [DOI] [PubMed] [Google Scholar]
- 8.Rinde LB, Smabrekke B, Mathiesen EB, et al. Ischemic stroke and risk of venous thromboembolism in the general population: The tromso study. J Am Heart Assoc. 2016;5(11):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piazza G, Goldhaber SZ, Kroll A, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with prior stroke. Clin Appl Thromb Hemost. 2014;20(1):43-49. [DOI] [PubMed] [Google Scholar]
- 10.Pongmoragot J, Rabinstein AA, Nilanont Y, et al. Pulmonary embolism in ischemic stroke: Clinical presentation, risk factors, and outcome. J Am Heart Assoc. 2013;2(6):e000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Zhang T, Zhou L, Yin X, Dong Q. Stratification of venous thromboembolism risk in stroke patients by caprini score. Ann Palliat Med. 2020;9(3):631-636. [DOI] [PubMed] [Google Scholar]
- 12.Cronin M, Dengler N, Krauss ES, et al. Completion of the updated caprini risk assessment model (2013 Version). Clin Appl Thromb Hemost. 2019 Jan-Dec;25:1076029619838052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36(8):1776-1781. [DOI] [PubMed] [Google Scholar]
- 14.Abdul Sultan A, West J, Stephansson O, et al. Defining venous thromboembolism and measuring its incidence using Swedish health registries: A nationwide pregnancy cohort study. BMJ Open. 2015;5(11):e008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Wang F, Xiong LL, Sun HY, Li W. The occurrence and risk factors of stroke complicated by venous thromboembolism. Sichuan Da Xue Xue Bao Yi Xue Ban. 2023;54(3):638-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis TC, Cortes J, Altshuler D, Papadopoulos J. Venous thromboembolism prophylaxis: A narrative review with a focus on the high-risk critically ill patient. J Intensive Care Med. 2019;34(11-12):877-888. [DOI] [PubMed] [Google Scholar]
- 17.Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019;94(7):833-839. [DOI] [PubMed] [Google Scholar]
- 18.Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017;70(19):2411-2420. [DOI] [PubMed] [Google Scholar]
- 19.Tondel BG, Morelli VM, Hansen JB, Braekkan SK. Risk factors and predictors for venous thromboembolism in people with ischemic stroke: A systematic review. J Thromb Haemost. 2022;20(10):2173-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migita S, Okumura Y, Fukuda I, et al. Relationship between baseline D-dimer and prognosis in Japanese patients with venous thromboembolism: Insights from the j'xactly study. Front Cardiovasc Med. 2023;10:1074661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang D, Li F, Du X, et al. Diagnostic accuracy of biomarker D-dimer in patients after stroke suspected from venous thromboembolism: A diagnostic meta-analysis. Clin Biochem. 2019;63:126-134. [DOI] [PubMed] [Google Scholar]
- 22.Kong XL, Zhang X, Zhang SJ, Zhang L. Plasma level of D-dimer is an independent diagnostic biomarker for deep venous thrombosis in patients with ischemic stroke. Curr Neurovasc Res. 2016;13(2):100-106. [DOI] [PubMed] [Google Scholar]
- 23.Corley AM, Sullivan MJ, Friedman SE, O'Rourke DJ, Palac RT, Gemignani AS. Relation of venous thromboembolism risk to ischemic stroke risk in hospitalized patients with cancer. Am J Cardiol. 2019;123(4):679-683. [DOI] [PubMed] [Google Scholar]
- 24.Li SY, Feng L, Xiao MJ, Chen SY, He JC, Wang Z. Derivation and validation of a clinical prediction scale for isolated distal deep venous thrombosis in patients after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(10):2087-2092. [DOI] [PubMed] [Google Scholar]
- 25.Bronic A, Coen Herak D, Margetic S, Milic M. Croatian Society of medical biochemistry and laboratory medicine: National recommendations for blood collection, processing, performance and reporting of results for coagulation screening assays prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen and D-dimer. Biochem Med (Zagreb). 2019;29(2):020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G, Favaloro EJ. Activated partial thromboplastin time: New tricks for an old dogma. Semin Thromb Hemost. 2008;34(7):604-611. [DOI] [PubMed] [Google Scholar]
- 27.White GC, 2nd. The partial thromboplastin time: Defining an era in coagulation. J Thromb Haemost. 2003;1(11):2267-2270. [DOI] [PubMed] [Google Scholar]
- 28.Dubey L, Dorosh O, Dubey N, et al. COVID-19-induced coagulopathy: Experience, achievements, prospects. Cardiol J. 2023;30(3):453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka KA, Terada R, Butt AL, Mazzeffi MA, McNeil JS. Factor VIII: A dynamic modulator of hemostasis and thrombosis in trauma. Anesth Analg. 2023;136(5):894-904. [DOI] [PubMed] [Google Scholar]
- 30.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu F, Wu Y, Zhang A, et al. Changes of coagulation function and risk of stroke in patients with COVID-19. Brain Behav. 2021;11(6):e02185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagraj S, Varrias D, Hernandez Romero G, et al. Incidence of stroke in randomized trials of COVID-19 therapeutics: A systematic review and meta-analysis. Stroke. 2022;53(11):3410-3418. [DOI] [PubMed] [Google Scholar]
- 33.Katsoularis I, Fonseca-Rodriguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modin D, Claggett B, Sindet-Pedersen C, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142(21):2080-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntaios G, Michel P, Georgiopoulos G, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: The global COVID-19 stroke registry. Stroke. 2020;51(9):e254-e2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020; 51(7):2002-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SM, Premraj L, Fanning J, et al. Ischemic and hemorrhagic stroke among critically ill patients with coronavirus disease 2019: An international multicenter coronavirus disease 2019 critical care consortium study. Crit Care Med. 2021;49(12):e1223-e1e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: A new challenge. Thromb Haemost. 2020;120(6):949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324(8):799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faura J, Bustamante A, Miro-Mur F, Montaner J. Stroke-induced immunosuppression: Implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. 2021;18(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. 2020;51(10):3156-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colling ME, Tourdot BE, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. 2021;128(12):2017-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinod K, Deppermann C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets. 2021;32(3):314-324. [DOI] [PubMed] [Google Scholar]
- 45.Ji R, Wang D, Shen H, et al. Interrelationship among common medical complications after acute stroke: Pneumonia plays an important role. Stroke. 2013;44(12):3436-3444. [DOI] [PubMed] [Google Scholar]
- 46.Suda S, Aoki J, Shimoyama T, et al. Stroke-associated infection independently predicts 3-month poor functional outcome and mortality. J Neurol. 2018;265(2):370-375. [DOI] [PubMed] [Google Scholar]
- 47.de Jonge JC, Takx RAP, Kauw F, de Jong PA, Dankbaar JW, van der Worp HB. Signs of pulmonary infection on admission chest computed tomography are associated with pneumonia or death in patients with acute stroke. Stroke. 2020;51(6):1690-1695. [DOI] [PubMed] [Google Scholar]
- 48.Kishore AK, Jeans AR, Garau J, et al. Antibiotic treatment for pneumonia complicating stroke: Recommendations from the pneumonia in stroke consensus (PISCES) group. Eur Stroke J. 2019;4(4):318-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hetze S, Engel O, Romer C, et al. Superiority of preventive antibiotic treatment compared with standard treatment of poststroke pneumonia in experimental stroke: A bed to bench approach. J Cereb Blood Flow Metab. 2013;33(6):846-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vermeij JD, Westendorp WF, Dippel DW, van de Beek D, Nederkoorn PJ. Antibiotic therapy for preventing infections in people with acute stroke. Cochrane Database Syst Rev. 2018;1(1):CD008530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarma PR. Red Cell Indices. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths;1990. [PubMed] [Google Scholar]
- 52.Onishi S, Ichiba T, Miyoshi N, Nagata T, Naito H. Unusual underlying disorder for pulmonary embolism: Cold agglutinin disease. J Cardiol Cases. 2017;15(2):43-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winningham MJ, Haussen DC, Nogueira RG, et al. Periprocedural heparin use in acute ischemic stroke endovascular therapy: The TREVO 2 trial. J Neurointerv Surg. 2018;10(7):611-614. [DOI] [PubMed] [Google Scholar]
- 54.Kay R, Wong KS, Yu YL, et al. Low-molecular-weight heparin for the treatment of acute ischemic stroke. N Engl J Med. 1995;333(24):1588-1593. [DOI] [PubMed] [Google Scholar]
- 55.Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8(1):10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296241254104 for Nomogram for Risk of Secondary Venous Thromboembolism in Stroke Patients: A Study Based on the MIMIC-IV Database by Folin Lan, Tianqing Liu, Celin Guan, Yufen Lin, Zhiqin Lin, Huawei Zhang, Xiaolong Qi, Xiaomei Chen and Junlong Huang in Clinical and Applied Thrombosis/Hemostasis