Abstract
Managing severe chronic pain is a challenging task, given the limited effectiveness of available pharmacological and non-pharmacological treatments. This issue continues to be a significant public health concern, requiring a substantial therapeutic response. Ziconotide, a synthetic peptide initially isolated from Conus magus in 1982 and approved by the US Food and Drug Administration and the European Medicines Agency in 2004, is the first-line intrathecal method for individuals experiencing severe chronic pain refractory to other therapeutic measures. Ziconotide produces powerful analgesia by blocking N-type calcium channels in the spinal cord, which inhibits the release of pain-relevant neurotransmitters from the central terminals of primary afferent neurons. However, despite possessing many favorable qualities, including the absence of tolerance development, respiratory depression, and withdrawal symptoms (largely due to the absence of a G-protein mediation mechanism), ziconotide's application is limited due to factors such as intrathecal administration and a narrow therapeutic window resulting from significant dose-related undesired effects of the central nervous system. This review aims to provide a comprehensive and clinically relevant summary of the literatures concerning the pharmacokinetics and metabolism of intrathecal ziconotide. It will also describe strategies intended to enhance clinical efficacy while reducing the incidence of side effects. Additionally, the review will explore the current efforts to refine the structure of ziconotide for better clinical outcomes. Lastly, it will prospect potential developments in the new class of selective N-type voltage-sensitive calcium-channel blockers.
Keywords: Pain management, N-type calcium channel blocker, Intrathecal administration strategies, Controversies
1. Introduction
Pain is a common reason for seeking medical attention [1,2]. It profoundly impacts individuals' quality of life and places a considerable social and economic burden on societies worldwide [[2], [3], [4], [5], [6]]. It is categorized based on its duration, with acute pain having a short-term nature and often being linked to known causes, such as exposure to heat, extreme cold, chemical irritants [7], and chronic pain which persists or recurs for a minimum of three months [2,[8], [9], [10]], affecting approximately 20 % of the global population [2,11]. Chronic pain's development and progression are influenced by various factors, and among these, cancer pain continues to represent a significant portion [12,13]. Pain can also be categorized by its nature: physiologic pain (a kind of chronic pain) and neuropathic pain [14,15]. In the 'Draft Guidelines for Chronic Pain' published by the National Institute for Health and Care Excellence (NICE), the recommended treatments for chronic pain include paracetamol and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for managing mild to moderate pain, while opioids are suggested for the treatment of chronic pain, particularly in the context of cancer-related pain [9]. Despite significant progress in analgesic management, many individuals continue to experience uncontrolled pain and intolerable side effects. Therefore, there is an ongoing need to prioritize the exploration of new analgesics and routes of administration in the field of pain management.
Voltage-Gated Calcium Channels (VDCCs) constitute the primary pathway through which calcium enters neurons during depolarization, contributing to the processing of pain signals. This is because the release of certain pain-related neurotransmitters, such as glutamate, Calcitonin Gene-Related Peptide (CGRP), and Substance P (SP), is dependent on calcium [7]. Based on the voltage dependence of activation, the calcium channel family is broadly divided into two subgroups: high-voltage-activated and low-voltage-activated channels. The latter group, characterized by their pharmacological and functional properties, is more diverse and can be further subdivided into L-, N-, P-, Q-, and R-type calcium channels [16]. N-type calcium channels, encoded by the Cav2.2 subunit [17,18], exist in various states including on, off, rest, and inactivation [19]. Furthermore, VGCCs, particularly the High Voltage-Activated (HVA) channels like N-type, are composed of three primary subunits: Cavα1, α2-δ, and β. The Cavα1 subunit serves as the pore-forming component, crucial for the formation of the channel's ion-conducting pore and the site where ziconotide exerts its action. The α2-δ subunit acts as an auxiliary structure, playing a pivotal role in channel trafficking and modulation of channel kinetics, whereas the β auxiliary subunit significantly contributes to the regulation of channel gating properties. They are predominantly expressed in neuronal tissues [20], particularly enriched at presynaptic nerve terminals. Here, they facilitate the release of neurotransmitters by physically interacting with the synaptic release machinery [21]. This mechanism also extends to terminals of primary afferent fibers, which form synapses localized in the dorsal horn of the spinal cord [22]. Hence, the inhibition of N-type channel activity by certain agents, like ω-conotoxin MVIIA, leading to reduced neurotransmission, suggests that N-type channels could be promising targets for novel analgesics [23].
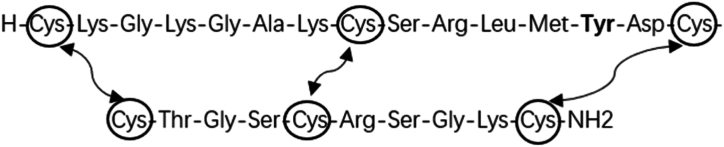
Ziconotide is a synthetic version (as depicted in Fig. 1) of the hydrophilic conopeptide ω-conotoxin MVIIA, originally isolated from the marine snail Conus magus in 1982 while it inhabited the Pacific Ocean [24]. The slow-paced Conus magus employs specific hunting strategies to capture fast-moving fish, slower-moving mollusks, and worms by shooting hollow radular teeth filled with poisonous peptides [25,26]. It is the inaugural member of a new drug class known for its potent inhibitory activity on N-type voltage-gated calcium channels [27], with minimal affinity for other ion channels and no interaction with cholinergic, monoaminergic, or peptidergic receptors [28]. In June 2000, Elan received approval from the US Food and Drug Administration (FDA) for the intrathecal injection of ziconotide as a pain medication, with the Phase III ischemia trial still ongoing [29]. In 2004, the FDA officially licensed ziconotide for intrathecal administration in the treatment of severe pain [30]. Ziconotide has a molecular formula of C102H172N36 O32S7, a relative molecular weight of 2639.12, a CAS chemical identifier number of 107452-89-1, and an isoelectric point of 11.2. The molecule consists of 25 highly hydrophilic amino acids arranged in the following sequence: H-Cys-Lys-Gly-Lys-Gly-Ala-Lys-Cys-Ser-Arg-Leu-Met-Tyr-Asp-Cys-Cys-Thr-Gly-Ser-Cys-Arg-Ser-Gly-Lys-Cys-NH2 cyclic (1–16), (8–20), (15–25)-tris(disulfide) (Fig. 2) [[31], [32], [33]]. These amino play a crucial role in stability of molecule [34] and includes essential amino acids, including four Lysine (Lys) and two Arginine (Arg) residues. Additionally, the molecule exists as a cation in bodily fluids. In non-in vivo settings, free l-methionine, which exists in the vials (50ug/ml), is used as the vehicle for ziconotide because it is more easily oxidized compared with the methionine in ziconotide.
Fig. 1.
The chemical structure of ziconotide.
Fig. 2.
Amino acid sequence of ziconotide. Amino acid sequence of ziconotide showed as standard three-letter amino acid code. The carboxyl terminus of ziconotide is amidated and both the N-terminal and C-terminal amino acids are cysteines that are covalently linked to two other, sequentially internal, cysteines via disulfide bridges which serve to stabilize the native conformation of the toxin and cause the peptide to display 4 loops, some of which contain important structural determinants of N-type calcium channel blocking activity, for example the tyrosine-13 (in bold font).
The 3-Dimensional (3D) solution structure has been determined using 2-Dimensional (2D) NMR spectroscopy [35], and its calcium channel binding pharmacophore was identified via structure-function analysis [36]. Structure-activity analysis has also revealed that the pharmacophores responsible for binding to calcium channels, specifically the Lys (Lysine) residues at position 2, Arg (Arginine) at positions 10 and 21, and the N-terminal, are crucial sites for the interaction between this molecule and N-type Voltage-Dependent Calcium Channels (VDCCs) [37]. Other residues important for selectivity and activity against the target ion channel include Leucine (Leu)11 [[36], [37], [38]]. Unlike opioids, which primarily act by binding to specific receptors or regulating receptor levels associated with pain, ziconotide alleviates pain by modulating synaptic function [39]. Specifically, ziconotide inhibits N-type calcium channels in the spinal cord, leading to reduced release of pain-relevant neurotransmitters in the central terminals of primary afferent neurons (Fig. 3) [[40], [41], [42]]. Due to its unique structure and acting sites, ziconotide cannot penetrate the Blood-Brain Barrier (BBB) [41,43,44], necessitating intrathecal administration as the approved route of delivery.
Fig. 3.
Mechanism of action of ziconotide. Ziconotide binds to N-type calcium channels located on the primary nociceptive afferent neurons in the superficial layers of the dorsal horn in the spinal cord. Ziconotide binding blocks calcium entry into the presynaptic nerve terminal, thereby reducing the release of excitatory neurotransmitters from the primary afferent nerves terminals into the synapse.
Ziconotide binds to N-type calcium channels located on the primary nociceptive afferent neurons in the superficial layers of the dorsal horn in the spinal cord. Ziconotide binding blocks calcium entry into the presynaptic nerve terminal, thereby reducing the release of excitatory neurotransmitters from the primary afferent nerves terminals into the synapse. Physically, ziconotide lodges into the permeation pathway of the channels as a pore blocker with a quite low unblocking rate [45].
Since Bier's pioneering cocainization of the spinal cord in 1898, many researchers have explored the epidural space as a viable administration route [46,47]. Intrathecal therapy offers a distinct advantage by allowing patients to use lower drug dosages, bypassing the BBB to enable direct medication delivery into the Cerebrospinal Fluid (CSF) and enhancing analgesic efficacy while reducing the occurrence of systemic adverse reactions often associated with oral administration [48]. Intrathecal therapy for chronic refractory pain management has been in use since the 1980s. Its adoption was driven by a deeper understanding of spinal anatomy and experiences gained in the fields of anesthesia and analgesia [49,50]. Intrathecal therapy remains a cornerstone in the current landscape, primarily because, to date, no alternative methods have demonstrated superior long-term efficacy [51]. It's worth noting that while the utilization of intrathecal therapy for chronic pain management has increased, thanks to the advent of programmable pumps and refined dose modulation techniques in recent years [52], it still stands as one of the last-resort options for pain management, as per pharmacological pain management guidelines [31].
2. Overview of the market and alternative therapies
It is widely recognized that, for patients experiencing severe pain, particularly those with terminal cancer, advancements in pain relief are of paramount importance. To date, in most countries, opioids like morphine, oxycodone, and hydromorphone are the most commonly employed medications for the management of severe chronic pain. However, a notable drawback of morphine is that as the dosage and duration of treatment increase, there is a higher risk of poor efficacy, tolerance, addiction, and respiratory depression [53]. Based on the unique mechanism of action described briefly above, ziconotide addresses the limitations of opioid painkillers, offering high potency without the concerns of tolerance and addiction. Presently, ziconotide is available in preservative-free isotonic solution formulations, including 1 mL, 2 mL, and 5 mL vials at a concentration of 100 μg/mL, as well as a 20 mL formulation at a concentration of 25 μg/mL. Storage is required at a constant temperature of 2–8 °C. Ziconotide is not approved for use in many countries, except for its licensing by the FDA in 2004 and the European Medicines Evaluation Agency (EMEA) in 2005, respectively. Consequently, there are limited products and information available regarding its marketing. It has not yet been routinely prescribed in some developed countries, such as England and the United States, with one concern raised is whether this allocation of resources represents the most optimal approach [6]. Moreover, the regular administration of ziconotide, either on its own or in combination with morphine, offers an alternative for a group of individuals who have limited pain management options. However, it's worth noting that the use of intrathecal ziconotide in conjunction with morphine does increase healthcare costs in England and the budget impact analysis does not strongly suggest that the incremental costs are substantial [6]. All of the information presented above points to the potential effectiveness of ziconotide in treating chronic pain, yet it's crucial to consider why it hasn't gained widespread application.
3. Pharmacokinetics and metabolism
3.1. Administration
3.1.1. Initiation
Ziconotide exclusively enters the nervous system through direct intrathecal administration. In clinical practice, it is administered either as a single injection or through continuous intrathecal infusion to evaluate the patient's tolerance to the dose and assess pain relief [54]. Typically, physicians adopt a strategy of starting with a 2 μg dose and monitoring the patient for 6–8 h. If the initial bolus does not provide adequate pain relief without adverse events, physicians often increase the bolus to 4 μg (with the maximum reported dose being 8 μg) at a later date and evaluate patients for an acceptable response, defined as a reduction in pain of at least 30 % with no unacceptable side effects [55].
3.1.2. Maintenance
Once the initial trial, which is only mandatory for the USA, has been deemed a success, it is advisable to implant a permanent device for stable continuous infusion. Based on the data obtained, experts recommend a minimum dose of 0.5–1.0 μg/day, with a compounding pharmacist diluting the drug to achieve this dosage. Alternatively, a more aggressive dose of 1.2 μg–2.4 μg/day, without the need for dilution, is considered acceptable. Dose adjustments can also be made based on the patients' evaluation, with a maximum recommended dose increase of 1.2 μg/day, following the FDA's specified maximum allowable dose of 19.2 μg/day [55]. Regardless of the chosen dose, it is essential to maintain a continuous and steady infusion rate. Recent recommendations suggest that initiating treatment with a lower dose, using smaller dose increments, and lengthening the intervals between dose adjustments can improve the safety of ziconotide administration, while also potentially providing economic benefits [6]. In summary, the approach with ziconotide is to "start low and go slow." A promising dose is one that leads to a reduction in pain of at least 30 % without causing undesirable side effects [55]. For the special touch, currently, adjusting the dose daily is considered too rapid, while administering it once a week is advisable. A quality reference could not be found to support this recommendation.
3.2. Pharmacokinetics
Ziconotide initially distributes mostly within the CSF, where it achieves nearly 100 % bioavailability and is not metabolized in the subarachnoid space [56]. It follows linear kinetics with low variability among cases, displaying a twofold range of 2.9–6.5 h and a median value of 4.5 h, whether administered as a single bolus or continuous infusion, and steady-state levels in the CSF can typically be attained within 24 h [56,57]. There is a slight discrepancy between the actual time required to reach the steady state and the theoretical estimate based on the half-life, which may be attributed to the absorption of ziconotide after it enters the Central Nervous System (CNS) [43,58]. There is no evidence to suggest that the pharmacokinetics of ziconotide in the CSF are linked to variables such as blood pressure, heart rate, respiratory rate, or body temperature [59].
3.2.1. Distribution
Ziconotide has a calculated apparent volume of distribution in the CSF that ranges from 34.4 to 1350 mL, with an overall median value of approximately 99 mL [56], suggesting that intrathecal ziconotide is primarily distributed within the CSF [56,57,60]. Furthermore, some studies have shown that even low doses of ziconotide can spread from the lumbar region to the brain and brainstem tissue [57,61]. The literatures also indicates that distribution of ziconotide is similar to that of other hydrophytic drugs [62,63]. The site of administration might not be a significant factor to consider when other conditions are matched abstractly; however, in 2017, PACC guidelines recommended placing the catheter tip to optimize the delivery of intrathecal analgesics at the site corresponding to the dermatomal distribution of the pain being treated [64,65].
3.2.2. Metabolism and elimination
It is suggested that the clearance of ziconotide occurs through physical transport, not chemical decomposition. The values of ziconotide's clearance are similar to the reports about CSF flow rates [66]. The clearance of ziconotide from lumber CSF shows significant interpatient variability [56], but the clearance rate is similar to the CSF turnover rate, which ranges from 0.3 to 0.4 mL/min [[67], [68], [69]]. During transit, ziconotide is initially cleaved by endopeptidases and exopeptidases into peptide fragments and free amino acids, which are then rapidly metabolized by peptidases and proteases in the systemic circulation [70]. Less than 1 % of the drug is excreted through the kidneys [43,71]. Only a minimal concentration of ziconotide can be detected in plasma after continuous high-dose administration of ziconotide for several days [56,59]. Currently, there is a severe lack of data regarding ziconotide metabolites in humans. This knowledge gap may stem from concerns that repeated procedures could increase the likelihood of patients experiencing more side effects or disadvantages without any benefits. This pertains to both infusing the drug and extracting CSF samples through the same catheter. Additionally, inserting two catheters or repeating punctures might disrupt the drug's kinetics and elevate the risk of infection. This is further compounded by the fact that ziconotide's concentration in plasma is too low to be effectively extracted and concentrated. Reports have suggested that ziconotide may be secreted through breast milk. However, specific and published information on this topic is currently lacking. As a result, it is advisable to administer ziconotide intrathecally under the guidance and supervision of healthcare professionals, as indicated in the package insert.
4. Indications
Ziconotide, as indicated by the FDA (Table 1), is approved for the treatment of patients experiencing chronic, severe pain that is either intolerant or refractory to systemic analgesics, adjunctive therapies, and other intrathecal treatments, including conditions such as cancer pain, HIV-related pain, neuropathic pain resulting from spinal cord injury, diabetic neuropathy, and others [72,73]. It's important to note that the indications for ziconotide should encompass conditions that specifically require intrathecal therapy since ziconotide can only be administered intrathecally. In clinical practice, three common factors justify intrathecal drug delivery: the drug's target is situated at the spinal level, the agent poorly penetrates the BBB when administered systemically, or the agent can penetrate the BBB, but systemic administration results in intolerable side effects [74,75]. Furthermore, intrathecal therapy is typically recommended for chronic pain patients only after they have undergone several unsuccessful conservative treatments and/or experienced unmanageable adverse effects from opioids and/or minimally invasive interventions, without new surgical indications, making it the final step in the treatment ladder for patients with chronic non-cancer pain when all other pharmaceutical and invasive alternatives have been explored and failed, except for spinal cord stimulation [76,77]. The Polyanalgesic Consensus Conference (PACC) has outlined specific indications for intrathecal therapy that partially overlap with those of ziconotide, encompassing conditions such as Failed Back Surgery Syndrome (FBSS), Complex Regional Pain Syndrome (CRPS), axial neck or back pain not amenable to surgical treatment, spinal cord injury, abdominal/pelvic pain, extremity pain, trunk pain resulting from postherpetic neuralgia or post-thoracotomy syndromes, cancer pain, and others [[77], [78], [79]]. Among these conditions, neuropathic and localized pain are well-suited for intrathecal therapy [77], whereas patients with nonlocalized pain tend to have a lower success rate with intrathecal treatment [64].
Table 1.
Indications.
| Groups related | Compendium | Examples | |
|---|---|---|---|
| Drug | Refractory to other analgesics | Chronic severe pain | Advanced cancer pain [6,40,55] |
| diabetic peripheral neuropathy (DPN) [72,73] | |||
| HIV [73], etc. | |||
| Neuropathic pain | Spinal cord injury [77] | ||
| Administration | Refractory to other administration methods | Applicative when other conservative or minimally invasive methods failed or experiencing untreatable side-effects | Cancer pain [40,55,65] |
| Spinal cord injury [80] | |||
| Abdominal/pelvic pain [[77], [78], [79]] | |||
| Extremity pain [79] | |||
| Trunk pain [79], etc. |
5. Contraindications (Table 2)
Table 2.
Contradictions.
| Groups related | Compendium | Examples |
|---|---|---|
| Ziconotide | Pre-existing history of psychiatric disorders | Depressive disorder [52,64] |
| Suicidal tendency [64,71] | ||
| Intrathecal administration | Patients suffering severe comorbidities | Psychiatric conditions [52] |
| Spinal infection or sepsis [81] | ||
| Coagulation disorders [52,82] | ||
| Other factors | Substandard medical conditions | Substandard aseptic surgical conditions [81] |
| Unpracticed faculties [83,84] |
Ziconotide has the potential to induce or exacerbate depression, especially in individuals at risk of suicide, making it unsuitable for patients with a history of psychosis [64]. It's important to strictly consider the contraindications of intrathecal therapy because ziconotide can only be administered in the subarachnoid space, either through a single injection or continuous administration via a pump. Given the invasive nature of intrathecal therapy, it is contraindicated in several cases including patients with severe medical comorbidities who are not surgical candidates, those with psychiatric disorders, individuals with spinal infections or sepsis, and patients taking anticoagulant medication who cannot adhere to the prescribed regimen during the perioperative period [52]. Additionally, the implantation of intrathecal catheters in anticoagulated patients should be avoided, except in rare instances [82]. Other situations where intrathecal therapy is not applicable include suboptimal medical conditions for the procedure, infection or inflammation at the puncture site, unmanageable bleeding factors, blocked CSF circulation resulting from spinal cord injury or spinal canal obstruction [81], and patients who are allergic to the solutions used [59]. Ziconotide is a peptide-based medication, which implies the potential for patient allergies, but fortunately, there has been no evidence of allergic or anaphylactic reactions reported in clinical practice thus far [42,57]. Furthermore, multiple procedures involving the subarachnoid space raise the risk of infection. Simultaneously, when chemotherapy is administered with an indwelling intrathecal catheter, it can alter CSF dynamics, resulting in unpredictable pharmacokinetics of ziconotide in the CSF, and intrathecal administration should be avoided when combined with intrathecal chemotherapy [43]. In particular, ziconotide is not recommended for use in children, adolescents, or pregnant women without medical advice [55]. Although there is limited information available on its clinical application during lactation, anecdotal evidence suggests that it is not a reason to discontinue breastfeeding when ziconotide is required by the mother [85]. However, the proper monitoring of breastfed infants is recommended for sedation because there is no clear evidence to confirm that ziconotide is not secreted through breast milk.
There are other important factors to consider when proceeding with intrathecal administration of ziconotide, such as a clear diagnosis, a strong social support system, and a life expectancy greater than three months, which should be determined before the implantation of a permanent intrathecal pump device [77]. In cases with shorter life expectancies, less than three months, the preferred approach is to use a subcutaneous port to connect to an intrathecal catheter [82]. Similarly, patients must exhibit a positive response to the treatment, any other condition necessitating surgery should be ruled out [77].
6. Clinical application and efficacy
6.1. Pharmacodynamic studies in human
In a wide range of animal pain models, intrathecal ziconotide produced potent antinociceptive effects, which were at least ten times more potent than those of intrathecal morphine [28,58,86]. Ziconotide was initially employed in humans for the treatment of severe pain in 1995 [43]. Over time, numerous trials examining the analgesic efficacy of ziconotide have been conducted and suggested that the analgesic effect of ziconotide is associated with the rate of titration and dosage [87]. To date, data on the analgesic efficacy of ziconotide in humans primarily rely on two trials [73,88] that employed a fast titration schedule, while one trial used a slower titration method [89]. The following trials were randomized, double-blind, placebo-controlled studies, and a morphine control group, and focused on patients experiencing severe, chronic pain unresponsive to standard treatments. In one study involving patients with various cancer types or AIDS, who were experiencing severe pain despite systemic or intrathecal analgesic regimens, ziconotide treatment led to a 53.1 % reduction in Visual Analogue Scale (VAS) pain intensity scores [73]. In another study, using a fast-dose titration, the ziconotide group exhibited a 30.7 % reduction in visual analogue pain scores among patients with chronic non-malignant pain of neuropathic or non-neuropathic origin, who had unsatisfactory responses to systemic opioid therapy [88]. Unfortunately, in the two trials employing a fast titration schedule [73,88], a significant number of patients experienced severe and persistent cognitive and neuropsychiatric adverse events, some of which led to hospitalization and may have contributed to three fatalities. In the trial conducted with a slow titration schedule [89], the frequency and severity of adverse events were notably lower compared to the fast titration schedules, but the average pain relief achieved was only moderate, amounting to less than 15 %. This suggests that a low dose combined with a slow titration regimen results in a reduced occurrence of adverse events but provides insufficient efficacy, whereas the opposite yields different outcomes [90,91].
A successful trial has been defined as achieving a pain reduction of at least 30 % with no unacceptable side effects, although there is debate among clinicians with some recommending a 50 % reduction as the required level [89]. These standards are currently in place, even though they can be complicated by the challenge many patients face in understanding the numerical pain scale and defining pain reduction by a percentage. While ziconotide is associated with a statistically significant analgesic effect, there is insufficient data to compare its antinociceptive efficacy with that of intrathecal morphine. The reasons for this lack of data could be attributed to the fact that these drugs take different approaches, the subjective nature of pain, and limitations in the trial population. Considering the analgesic efficacy and adverse effects associated with other schedules, the FDA and EMEA have, so far, only approved the regimen of slow titration for ziconotide.
6.2. Other clinical applications
Ziconotide has also shown efficacy in specific diseases. For example, it was employed in cases of refractory primary erythromelalgia after other pharmacological treatments had been attempted and failed, including local anesthetics, capsaicin, and dantrolene, all of which provided only 24–48 h of relief followed by symptom recurrence [92]. Reports have suggested the intrathecal administration of a low dose of ziconotide in individuals with lower limb spasticity after finding intrathecal baclofen to be ineffective [93]. It has also been used for severe spasticity following spinal cord injury [80]. It's worth noting that the conclusions from these reports do not perfectly align with the idea that the efficacy of ziconotide in the treatment of refractory spasticity is ideal [43]. A comatose patient with a spinal cord injury was initially treated with intrathecal baclofen to control spasms but remained comatose. The gradual recovery of consciousness occurred upon the addition of intrathecal ziconotide. Unfortunately, the patient fell back into a coma after discontinuing ziconotide. This case suggests that ziconotide may be a viable candidate for its synergistic antispastic effects when combined with baclofen in patients with an implanted intrathecal pump [94]. Intrathecal ziconotide therapy was also reported to effectively treat chronic refractory migraines [70]. Another finding suggests that intrathecal ziconotide can improve emotional components and function while relieving pain [95]. This finding appears to be in contrast to the opinion that ziconotide has the potential to induce or exacerbate depression. Considerations should be given to whether the enhanced mood status in a very limited number of cases resulted from pain relief rather than ziconotide itself. Additionally, there is controversy surrounding ziconotide's effectiveness in acute pain [43,82]. To resolve these contradictions, there is an urgent need for additional clinical data.
In certain training studies related to the management of refractory pain, off-label use of medications and combination therapies have been reported. The theoretical basis for this approach lies in the idea that different drugs target distinct mechanisms, potentially maximizing efficacy while minimizing side effects. For instance, enhanced analgesic effects were observed when ziconotide and intrathecal morphine were combined [53,96], validating earlier findings from animal experiments [72,97]. A patient with breast cancer received the same therapy mentioned earlier after other treatments had failed, resulting in effective pain control but accompanied by symptoms of nausea, vomiting, and CSF leakage confirmed by Computerized Tomography (CT), which was successfully treated with a blood patch [98]. In a clinical trial, a triple intrathecal therapy combining ziconotide, morphine, and levobupivacaine was found to be effective in patients with end-stage malignant pain who were unresponsive to high doses of systemic opioids, allowing rapid pain control and resulting in a significant reduction in the VAS score within 48 h, leveraging the approach of utilizing different drugs acting on distinct receptors [99]. Pharmacokinetic drug-drug interaction studies in this context are notably lacking, and it's important to note that the FDA and EMA have approved intrathecal therapy using morphine and ziconotide as monotherapies. Consequently, the combination therapy of intrathecal morphine, ziconotide, and other agents is considered off-label. The PACC recommends refraining from off-label intrathecal monotherapy until FDA-approved drugs have been attempted and demonstrated to be ineffective [82].
Intrathecal drug delivery is typically considered a last resort for managing chronic intractable pain, and it's essential to note that ziconotide is exclusively approved as intrathecal monotherapy [31]. Many patients have previously received other forms of medical administration, so challenges can arise when transitioning from pretreated intrathecal opioids to intrathecal ziconotide. At present, there is no well-established theoretical protocol or approach for converting intrathecal opioid doses into equivalent oral or intravenous doses, let alone transitioning directly to intrathecal ziconotide. A recommended strategy is to gradually taper off intrathecal opioid infusion over several weeks and replace it with an oral or intravenous alternative, as this approach helps mitigate or prevent withdrawal symptoms while maintaining adequate analgesia [100,101]. Both gabapentin and ziconotide are prominent calcium channel-targeted drugs, although the comparison between their efficacy is notably limited [102].
7. Safety and tolerability
The use of ziconotide is limited due to its inconvenient administration, narrow therapeutic range, delayed onset of action, slow response to dose adjustments, and dose-related side effects (Table 3).
Table 3.
Complications.
| Groups related | Compendium | Examples |
|---|---|---|
| Ziconotide | Mental &nervous system | Poor analgesic efficacy [103,104] |
| Depression [105,106] | ||
| Aggravate suicidal tendency [106] | ||
| Somnolence [91,80] | ||
| Blurred vision | ||
| Motor system | Dysarthria and shiver | |
| Vertigo [80], etc. | ||
| Circulatory system | Hypotension [55,103] | |
| Peripheral edema [103] | ||
| Digestive system | Worsen morphine-induced constipation [40] | |
| Nausea [91] | ||
| Diarrhea [91,80] | ||
| Urinary system | Impaired Renal function? [58] | |
| Procedure | vascular injury [59], local infection, flaccid paraplegia/paraplegia [70,82], leakage of CSF [56,58], meningitis [82], spinal hematoma [77] and so on. | |
| Device | Catheter dislocation &leakage [107], pump malfunction [[108], [109], [110]], etc. | |
7.1. Medication-related information
Ziconotide, administered via continuous intrathecal infusion, appears to maintain its analgesic efficacy for several months without causing permanent adverse effects [42,60]. Nevertheless, it's important to note that toxicity has been linked to the dose and rate of infusion, which restricts the application of ziconotide [6]. An additional analysis of ziconotide's safety and efficacy revealed that achieving a higher CSF concentration 4 h after intrathecal infusion resulted in a greater analgesic effect but also correspondingly increased the risk of side-effects [91]. Common adverse events associated with ziconotide are generally not life-threatening and include peripheral edema, constipation, diarrhea, nausea, dizziness, blurred vision, somnolence, ataxia, headache, vertigo, dysarthria, and urinary retention [91,80]. Studies have indicated a higher incidence of psychiatric symptoms with intrathecal ziconotide, with 42 % of cases occurring in individuals over 65 years old compared to 29 % in those younger than 65 years old [40]. In contrast to opioids, which can lead to unexpected events such as respiratory depression, tolerance, and dependence, or the potential development of spinal catheter-tip granulomas associated with intrathecal morphine or hydromorphone use, ziconotide has not been reported to cause substantial systemic endocrine, hematological, or metabolic side effects [42,82]. It's important to note that ziconotide has the potential to induce or exacerbate depression and increase the risk of suicide in susceptible patients [105,106]. So far, no direct fatalities have been attributed to ziconotide, even in instances of extreme overdose [55]. Comprehensive psychological evaluations are strongly recommended for all patients before initiating ziconotide treatment [111].
During clinical studies, approximately one-tenth of the patients (with most of them participating in open-label trials) reported Creatinine Kinase (CK) concentrations exceeding three times the upper limit of normal [58]. It is unclear whether this increase is solely due to ziconotide therapy or if it might be influenced by other drugs, such as statins, known to elevate CK concentrations. There is typically no need for dose adjustment in patients with impaired liver and kidney function because ziconotide is a peptide that does not undergo phase I biotransformation or phase III conjugation reactions unless a specific organ insufficiency affects CSF clearance of ziconotide [56,58,60]. Monitoring CK levels and organ functions is recommended for security reasons, especially because there is limited formal research on medication administration in individuals with hepatic or renal insufficiency. Some articles demonstrated that ziconotide may potentially cause severe hypotension, although the exact mechanism remains unclear [55,103].
Many of the side effects, including cognitive and neuropsychiatric effects linked to rapid titration, can often be managed by reducing the dose [43,91]. Psychiatric symptoms can be treated with neuroleptics as a symptomatic approach [112,113]. Importantly, ziconotide can be discontinued immediately without causing withdrawal reactions if its mechanism of action is not G-protein mediated [30]. However, discontinuation can lead to poor pain control [89,91]. When patients anticipate unwanted effects, it's essential to consider dose adjustments or temporarily pausing the ziconotide infusion to prevent them from occurring [114]. Ziconotide has slow clearance, and its adverse effects may take time to resolve after a dose reduction or when the delivery is stopped [71]. Regrettably, there are few specific pharmacological antagonists available to counteract the toxicity of ziconotide. In such cases, it is advisable to admit the patient to the hospital promptly, provide hemodynamic support, and immediately stop the pump infusion [55]. Moreover, it's essential to consider the following points diligently: large, simple, and long-term follow-up studies are scarce, particularly among patients with advanced cancers and limited life expectancy; some trials have shown that patients reached the recommended maximum dose while tolerating side effects after slow titration over several weeks [42,53,115], although the reason for this phenomenon remains unclear; in a small subset of these patients who are otherwise refractory to treatment and suffer from severe chronic pain, slowly titrated ziconotide has led to complete pain relief and an improvement in their quality of life, highlighting the potential for significant individual-level benefits [57,70]. Further research is necessary to explore different therapeutic schedules and their potential advantages [55].
Several factors could contribute to these adverse effects. Firstly, the precise roles and distributions of each channel subtype are dependent on the specific type of neurons they are found in, and these channels support a wide range of functions [116]. The various functional roles of these channels present a challenge when developing new calcium channel-based therapeutics with a low risk of side effects. N-VDCCs are found throughout the thalamus, medulla, and epencephalon [113], and blocking them with ziconotide is non-state selective and reversible [19], which may lead to symptoms such as ataxia and shivering [117]. Secondly, a recent study suggested that the side effects of ziconotide may be linked to Met12 in loop 2 [118], although the exact mechanism remains unclear. Since ziconotide is a polypeptide, it carries inherent disadvantages associated with peptide compounds, such as the potential to induce inflammation or trigger an immune response via mast cell degranulation [119], though such reports have been infrequent.
7.2. Procedure and device-related information
The intrathecal delivery is not without its risks [59]. Reportedly, infection occurs in 2.4 %–3.6 % of cases with malignant tumors after the implantation of intrathecal drug devices, which might be associated with immunosuppression and anti-tumor therapy [120]. In case of central infection, removal of the intrathecal device is necessary [83]. In comparison to other intrathecal analgesics, a similar incidence of meningitis, which is more likely to be associated with the use of a subcutaneously implanted pump, was reported with ziconotide [60]. This suggests that ziconotide itself does not inherently increase the risk of meningitis [70,82]. Although the 2017 PACC guidelines strongly endorse a consensus, that the placement of the catheter tip should be close to the spinal level corresponding to the dermatome associated with the pain being treated with morphine [64]. This procedure is not a concern for ziconotide because it can rapidly spread to brain and brainstem tissue within minutes, even with a low dose injected from the lumbar region [57].
Other complications, including bleeding at the insertion site, epidural or subdural abscess, spinal hematoma and so on carry an intermediate risk and necessitate early surgical evacuation [77]. Preventing CSF leakage is also crucial, as indicated by some reported cases [98,121]. Repeated intrathecal injections and overly deep intrathecal punctures can directly lead to limb flaccid paraplegia, which may manifest as either reversible or irreversible paraplegia [122]. Fortunately, the risks associated with these complications are low [65]. For instance, performing these procedures under ultrasound guidance can reduce the associated risks of these procedures [82].
Hardware-related complications are relatively rare but can be life-threatening [107]. Both ambient temperature and the patient's body temperature, as well as factors like altitude (such as when traveling on a plane or at high altitudes), and the intensity of illumination during transportation and storage can have an impact on the stability of the device, thereby indirectly affecting the stability of ziconotide [108,109]. To prevent overdose, it is recommended that the patient's body temperature does not exceed 39 °C [110]. Magnetic fields, even the magnetic of laptop speakers can impact the stability of devices, resulting in poor pain control [123].
A thorough medical history review is essential for identifying potential complicating factors in candidate patients. Adverse effects typically manifest within 3–9 days but may persist for weeks after discontinuing the drug [71]. While intrathecal therapy presents a viable and relatively safe approach for treating both cancer- and noncancer-related pain, the process of trialing intrathecal drug delivery systems continues to be a subject of ongoing debate [78] Therefore, continuous updating of knowledge is is essential in this field [79].
8. Enhancing the role of the healthcare team
Successful treatment depends on careful patient selection, continuous monitoring of both effectiveness and potential side effects and the ability to adjust drug selection and dosage, which should be managed by healthcare professionals or multidisciplinary teams [84]. Pain specialists, anesthesiologists, neurosurgeons, oncologists, psychiatrists, physician assistants, nurse practitioners, and registered nurses are all invaluable members of intrathecal pain management teams, as they bring their professional expertise to pain management services [84,124]. Specifically, psychological evaluations are beneficial before and during the period of ziconotide medication, and even after discontinuation [111]. This involves regular monitoring of patients for cognitive impairment and changes in mood or consciousness [125], although the exact interval or frequency of these assessments is not clearly defined. A personalized treatment regimen, including an evaluation by pain specialists and neurosurgeons, before applying ziconotide is valuable, especially for patients dealing with chronic pain who have likely already undergone various patient-driven and medically-instructed treatments [77]. In cases where some patients, transitioning from opioids to ziconotide, experience emotional distress and develop psychological symptoms, express reservations about ziconotide, and wish to discontinue its use, a multimodal approach is recommended. This approach should encompass education about opioid withdrawal, psychological interventions like cognitive-behavioral coping strategies or relaxation techniques, the use of adjunct drugs to alleviate emotional issues, and maintaining close contact with treating physicians [84]. Primary care physicians are crucial in the process, as they play essential roles in patient selection and referral, providing ongoing collaborative care that includes monitoring for treatment efficacy and adverse events, also, facilitating communication with the treating specialist, leading to improved outcomes and patient satisfaction [124]. It is strongly recommended to monitor the patient's temperature in detail as it indirectly affects the stability of intrathecal devices, which helps avoid drug overdose [126]. During the trial, ziconotide may cause hypotension, so it's advisable to have a peripheral intravenous line to ensure adequate hydration and maintain hemodynamics [55,103]. However, in cases where there is no evidence of respiratory depression, patients can typically be discharged on the same day following a thorough medical evaluation [103]. A notable limitation is that many oncologists are not fully aware of the advantages of intrathecal drug delivery [127]. This knowledge gap exists because most of the literature and academic presentations on intrathecal drug delivery are outside the realm of oncology [127]. In conclusion, healthcare professionals involved in the entire treatment process should possess comprehensive knowledge of the devices and their management, be well-versed in the physicochemical properties of drugs delivered by the equipment, and be prepared to address potential complications. This multifaceted understanding is essential to guarantee the safety and optimize the prognosis of patients.
9. Future prospective
Efforts are underway to enhance the pharmaceutical properties of ziconotide for intravenous, oral, or transdermal administration to broaden the therapeutic window and simplify its use [55,128]. Subtle structural modifications have mainly focused on preserving the overall structure of ziconotide. Compared to their linear counterparts, cyclic peptides are typically more stable due to their head-to-tail cyclized backbone, providing them with resistance to adverse physiological conditions and protecting against degradation by various proteases [129]. This strategy can help enhance the biopharmaceutical properties of peptides [130,131]. The conventional approach for creating cyclic peptides involves constructing a linear precursor through Solid-Phase Peptide Synthesis (SPPS) [132]. Another method to generate cyclic analogs of ziconotide is by utilizing Asparaginyl Endopeptidase (AEP)-mediated cyclization [119]. The Borneol (BOR)-modified Liposomes (LIPs) were membrane fused with exosome Microneedles (MNs) in an animal study provided a safe and effective administration of chronic pain treatment by improving the ability of ziconotide across the BBB [133]. Myristoylated N-terminal ziconotide can undergo self-assembly onto micelles, resulting in extended analgesic effects and a notable reduction in the risk of side effects such as tremors and impaired motor coordination in mice [104]. Numerous patents have been filed to theoretically modify ziconotide's structure to enhance its ability to cross the BBB, but none of these modifications have been validated in humans or made commercially available [128,134]. Leconotide, however, when administered non-intrathecally, was denied approval due to the serious complications that emerged during phase II clinical trials [135].
In 2004, a pivotal event occurred with the FDA's approval of the first peptide toxin agent, ziconotide, marking a turning point in the field of analgesics, which led to a shift in research focus and sustained interest in exploring and discovering new analgesics derived from natural sources or synthesized compounds that modulate or block pain-associated ion channels [136,137]. Peptides derived from animal venoms, including those from cobras, cone snails, and spiders, can modulate or block voltage-gated calcium channels, leading to antinociceptive effects in various pain models, such as acute, inflammatory, neuropathic, postoperative, facial, visceral, and cancer-related pain [2,137].
As previously mentioned, ziconotide's non-selective and state-independent blocking of N-type Voltage-Dependent Calcium Channels (N-VDCCs) may contribute to its side effects. Therefore, further research into state-dependent blockers and specific calcium channel sites appears promising for enhancing the therapeutic index of ziconotide and minimizing related undesirable effects [[138], [139], [140]]. Several articles have demonstrated the gratifying chemical stability of various mixed combinations of agents, including morphine, ropivacaine, and ziconotide in syringes or polyolefin infusion bags [109,141]. These findings offer valuable insights into the potential for combined drug administration and multimodal analgesia, making combination drug therapy a promising avenue for exploration in both novel drug development and clinical practice.
10. Conclusion
Ziconotide boasts several advantageous features, including potent analgesic effects without the drawbacks of physical or psychological addiction and respiratory depression. These characteristics make it an appealing option for managing intractable pain, despite its limitations, such as a narrow therapeutic range, the need for intrathecal administration, a slow onset of action, and potential psychiatric side effects. The development of ziconotide illustrates the challenges associated with creating peptide-based therapeutics. Simultaneously, it underscores the unique mechanism of action, serving as a foundation for the development of peptides isolated from animal venoms or their synthetic counterparts, which modulate or block voltage-gated calcium channels [2], as well as non-peptide small molecules that can be taken orally [57]. These endeavors hold promise for achieving enhanced effectiveness and safety in the future.
Funding
This work was supported by the Natural Science Foundation of China (No. 42176098) and the High-Level Talents Special Program of Zhejiang (No. 2022R52036).
Institutional review board statement
not applicable.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Jinping Lin: Writing – original draft, Project administration, Conceptualization. Shuwei Chen: Writing – original draft. Usman Dawood Butt: Writing – review & editing. Min Yan: Writing – review & editing, Validation, Supervision. Bin Wu: Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Min Yan, Email: zryanmin@zju.edu.cn.
Bin Wu, Email: wubin@zju.edu.cn.
References
- 1.Loeser J.D., Treede R.D. The kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Trevisan G., Oliveira S.M. Animal venom peptides cause antinociceptive effects by voltage-gated calcium channels activity blockage. Curr. Neuropharmacol. 2022;20:1579–1599. doi: 10.2174/1570159X19666210713121217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breivik H., Cherny N., Collett B., de Conno F., Filbet M., Foubert A.J., Cohen R., Dow L. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann. Oncol. 2009;20:1420–1433. doi: 10.1093/annonc/mdp001. [DOI] [PubMed] [Google Scholar]
- 4.Singh H., Banipal R., Singh B. Assessment of adequacy of pain management and analgesic use in patients with advanced cancer using the brief pain inventory and pain management index calculation. J Glob Oncol. 2017;3:235–241. doi: 10.1200/JGO.2016.004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker S., Navratilova E., Nees F., Van Damme S. Emotional and motivational pain processing: current state of knowledge and perspectives in translational research. Pain Res. Manag. 2018;2018:1–12. doi: 10.1155/2018/5457870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambe T., Duarte R., Eldabe R., Copley S., Kansal A., Black S., Dupoiron D., Eldabe S. Ziconotide for the management of cancer pain: a budget impact analysis. Neuromodulation: Technology at the Neural Interface. 2023;26:1226–1232. doi: 10.1016/j.neurom.2022.08.458. [DOI] [PubMed] [Google Scholar]
- 7.Bourinet E., Altier C., Hildebrand M.E., Trang T., Salter M.W., Zamponi G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014;94:81–140. doi: 10.1152/physrev.00023.2013. [DOI] [PubMed] [Google Scholar]
- 8.Maniadakis N., Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95–103. doi: 10.1016/S0304-3959(99)00187-6. [DOI] [PubMed] [Google Scholar]
- 9.Shaheed C.A., Machado G.C., Underwood M. Drugs for chronic pain. Br. J. Gen. Pract. 2020;70:576–577. doi: 10.3399/bjgp20X713549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossipov M.H., Dussor G.O., Porreca F. Central modulation of pain. J. Clin. Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gureje O., Von Korff M., Simon G.E., Gater R. Persistent pain and well-being: a world health organization study in primary care. JAMA, J. Am. Med. Assoc. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 12.Reis-Pina P., Lawlor P.G., Barbosa A. Adequacy of cancer-related pain management and predictors of undertreatment at referral to a pain clinic. J. Pain Res. 2017;10:2097–2107. doi: 10.2147/JPR.S139715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snijders R., Brom L., Theunissen M., van den Beuken-van E.M. Update on prevalence of pain in patients with cancer 2022: a systematic literature review and meta-analysis. Cancers. 2023:15. doi: 10.3390/cancers15030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treede R.D., Jensen T.S., Campbell J.N., Cruccu G., Dostrovsky J.O., Griffin J.W., Hansson P., Hughes R., Nurmikko T., Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. NEUROLOGY. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 15.Jamison R.N., Rudy T.E., Penzien D.B., Mosley T.J. Cognitive-behavioral classifications of chronic pain: replication and extension of empirically derived patient profiles. Pain. 1994;57:277–292. doi: 10.1016/0304-3959(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 16.Bean B.P. Pharmacology of calcium channels in cardiac muscle, vascular muscle, and neurons. Am. J. Hypertens. 1991;4:406S–411S. doi: 10.1093/ajh/4.7.406s. [DOI] [PubMed] [Google Scholar]
- 17.Altier C., Dale C.S., Kisilevsky A.E., Chapman K., Castiglioni A.J., Matthews E.A., Evans R.M., Dickenson A.H., Lipscombe D., Vergnolle N., Zamponi G.W. Differential role of N-type calcium channel splice isoforms in pain. J. Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade A., Denome S., Jiang Y.Q., Marangoudakis S., Lipscombe D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat. Neurosci. 2010;13:1249–1256. doi: 10.1038/nn.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristipati R., Nadasdi L., Tarczy-Hornoch K., Lau K., Miljanich G.P., Ramachandran J., Bell J.R. Characterization of the binding of omega-conopeptides to different classes of non-L-type neuronal calcium channels. Mol. Cell. Neurosci. 1994;5:219–228. doi: 10.1006/mcne.1994.1026. [DOI] [PubMed] [Google Scholar]
- 20.Nowycky M.C., Fox A.P., Tsien R.W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 21.Zamponi G.W. Regulation of presynaptic calcium channels by synaptic proteins. J. Pharmacol. Sci. (Tokyo, Jpn.) 2003;92:79–83. doi: 10.1254/jphs.92.79. [DOI] [PubMed] [Google Scholar]
- 22.Gruner W., Silva L.R. Omega-conotoxin sensitivity and presynaptic inhibition of glutamatergic sensory neurotransmission in vitro. J. Neurosci. 1994;14:2800–2808. doi: 10.1523/JNEUROSCI.14-05-02800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagarajan S., Chakravarty P.K., Park M., Zhou B., Herrington J.B., Ratliff K., Bugianesi R.M., Williams B., Haedo R.J., Swensen A.M., Warren V.A., Smith M., Garcia M., Kaczorowski G.J., McManus O.B., Lyons K.A., Li X., Madeira M., Karanam B., Green M., Forrest M.J., Abbadie C., McGowan E., Mistry S., Jochnowitz N., Duffy J.L. A potent and selective indole N-type calcium channel (Ca(v)2.2) blocker for the treatment of pain. Bioorg. Med. Chem. Lett. 2011;21:869–873. doi: 10.1016/j.bmcl.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 24.McIntosh M., Cruz L.J., Hunkapiller M.W., Gray W.R., Olivera B.M. Isolation and structure of a peptide toxin from the marine snail Conus magus. Arch. Biochem. Biophys. 1982;218:329–334. doi: 10.1016/0003-9861(82)90351-4. [DOI] [PubMed] [Google Scholar]
- 25.Olivera B.M., Seger J., Horvath M.P., Fedosov A.E. Prey-capture strategies of fish-hunting cone snails: behavior, neurobiology and evolution. Brain Behav. Evol. 2015;86:58–74. doi: 10.1159/000438449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen L.T.T., Craik D.J., Kaas Q. Bibliometric review of the literature on cone snail peptide toxins from 2000 to 2022. Mar. Drugs. 2023;21:154. doi: 10.3390/md21030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morsy M.A., Gupta S., Dora C.P., Jhawat V., Dhanawat M., Mehta D., Gupta K., Nair A.B., El-Daly M. Venoms classification and therapeutic uses: a narrative review. Eur. Rev. Med. Pharmacol. Sci. 2023;27:1633–1653. doi: 10.26355/eurrev_202302_31408. [DOI] [PubMed] [Google Scholar]
- 28.Bowersox S.S., Luther R. Pharmacotherapeutic potential of omega-conotoxin MVIIA (SNX-111), an N-type neuronal calcium channel blocker found in the venom of Conus magus. Toxicon. 1998;36:1651–1658. doi: 10.1016/s0041-0101(98)00158-5. [DOI] [PubMed] [Google Scholar]
- 29.Jain K.K. An evaluation of intrathecal ziconotide for the treatment of chronic pain. Expet Opin. Invest. Drugs. 2000;9:2403–2410. doi: 10.1517/13543784.9.10.2403. [DOI] [PubMed] [Google Scholar]
- 30.Pope J.E., Deer T.R. Ziconotide: a clinical update and pharmacologic review. Expet Opin. Pharmacother. 2013;14:957–966. doi: 10.1517/14656566.2013.784269. [DOI] [PubMed] [Google Scholar]
- 31.Wermeling D.P. Ziconotide, an intrathecally administered N-type calcium channel antagonist for the treatment of chronic pain. Pharmacotherapy. 2005;25:1084–1094. doi: 10.1592/phco.2005.25.8.1084. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y., Harvey P.J., Koehbach J., Chan L.Y., Jones A., Andersson Å., Vetter I., Durek T., Craik D.J. A chemoenzymatic approach to produce a cyclic analogue of the analgesic drug MVIIA (ziconotide) Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202302812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen K.J., Thomas L., Lewis R.J., Alewood P.F., Craik D.J. A consensus structure for omega-conotoxins with different selectivities for voltage-sensitive calcium channel subtypes: comparison of MVIIA, SVIB and SNX-202. J. Mol. Biol. 1996;263:297–310. doi: 10.1006/jmbi.1996.0576. [DOI] [PubMed] [Google Scholar]
- 34.Rashid M., Manivet P., Nishio H., Pratuangdejkul J., Rajab M., Ishiguro M., Launay J.M., Nagatomo T. Identification of the binding sites and selectivity of sarpogrelate, a novel 5-HT2 antagonist, to human 5-HT2A, 5-HT2B and 5-HT2C receptor subtypes by molecular modeling. Life Sci. 2003;73:193–207. doi: 10.1016/s0024-3205(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 35.Basus V.J., Nadasdi L., Ramachandran J., Miljanich G.P. Solution structure of omega-conotoxin MVIIA using 2D NMR spectroscopy. FEBS Lett. 1995;370:163–169. doi: 10.1016/0014-5793(95)00819-u. [DOI] [PubMed] [Google Scholar]
- 36.Nadasdi L., Yamashiro D., Chung D., Tarczy-Hornoch K., Adriaenssens P., Ramachandran J. Structure-activity analysis of a Conus peptide blocker of N-type neuronal calcium channels. Biochemistry. 1995;34:8076–8081. doi: 10.1021/bi00025a013. [DOI] [PubMed] [Google Scholar]
- 37.Kandabashi T., Shimokawa H., Mukai Y., Matoba T., Kunihiro I., Morikawa K., Ito M., Takahashi S., Kaibuchi K., Takeshita A. Involvement of rho-kinase in agonists-induced contractions of arteriosclerotic human arteries. Arterioscler. Thromb. Vasc. Biol. 2002;22:243–248. doi: 10.1161/hq0202.104274. [DOI] [PubMed] [Google Scholar]
- 38.Gao S., Yao X., Yan N. Structure of human Ca(v)2.2 channel blocked by the painkiller ziconotide. Nature. 2021;596:143–147. doi: 10.1038/s41586-021-03699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGivern J.G. Ziconotide: a review of its pharmacology and use in the treatment of pain. Neuropsychiatric Dis. Treat. 2007;3:69–85. doi: 10.2147/nedt.2007.3.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banik R.K., Engle M.P. Ziconotide for management of cancer pain refractory to pharmacotherapy: an update. Pain Med. 2020;21:3253–3259. doi: 10.1093/pm/pnaa251. [DOI] [PubMed] [Google Scholar]
- 41.Skov M.J., Beck J.C., de Kater A.W., Shopp G.M. Nonclinical safety of ziconotide: an intrathecal analgesic of a new pharmaceutical class. Int. J. Toxicol. 2007;26:411–421. doi: 10.1080/10915810701582970. [DOI] [PubMed] [Google Scholar]
- 42.Wallace M.S., Rauck R., Fisher R., Charapata S.G., Ellis D., Dissanayake S. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth. Analg. 2008;106:628–637. doi: 10.1213/ane.0b013e3181606fad. [DOI] [PubMed] [Google Scholar]
- 43.Williams J.A., Day M., Heavner J.E. Ziconotide: an update and review. Expet Opin. Pharmacother. 2008;9:1575–1583. doi: 10.1517/14656566.9.9.1575. [DOI] [PubMed] [Google Scholar]
- 44.Skov M.J., Beck J.C., de Kater A.W., Shopp G.M. Nonclinical safety of ziconotide: an intrathecal analgesic of a new pharmaceutical class. Int. J. Toxicol. 2007;26:411–421. doi: 10.1080/10915810701582970. [DOI] [PubMed] [Google Scholar]
- 45.Feng Z.P., Hamid J., Doering C., Bosey G.M., Snutch T.P., Zamponi G.W. Residue Gly1326 of the N-type calcium channel alpha 1B subunit controls reversibility of omega-conotoxin GVIA and MVIIA block. J. Biol. Chem. 2001;276:15728–15735. doi: 10.1074/jbc.M100406200. [DOI] [PubMed] [Google Scholar]
- 46.Ericson T., Singla P., Kohan L. Intrathecal Pumps, Physical Medicine and Rehabilitation Clinics of North America. 2022;33:409–424. doi: 10.1016/j.pmr.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Belverud S., Mogilner A., Schulder M. Intrathecal pumps. Neurotherapeutics. 2008;5:114–122. doi: 10.1016/j.nurt.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatia G., Lau M.E., Koury K.M., Gulur P. Intrathecal Drug Delivery (ITDD) systems for cancer pain. F1000Res. 2013;2:96. doi: 10.12688/f1000research.2-96.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onofrio B.M., Yaksh T.L., Arnold P.G. Continuous low-dose intrathecal morphine administration in the treatment of chronic pain of malignant origin. Mayo Clin. Proc. 1981;56:516–520. [PubMed] [Google Scholar]
- 50.Coombs D.W., Fine N. Spinal anesthesia using subcutaneously implanted pumps for intrathecal drug infusion. Anesth. Analg. 1991;73:226–231. doi: 10.1213/00000539-199108000-00019. [DOI] [PubMed] [Google Scholar]
- 51.De Andres J., Rubio-Haro R., De Andres-Serrano C., Asensio-Samper J.M., Fabregat-Cid G. Intrathecal drug delivery. Methods Mol. Biol. 2020;2059:75–108. doi: 10.1007/978-1-4939-9798-5_3. [DOI] [PubMed] [Google Scholar]
- 52.Bottros M.M., Christo P.J. Current perspectives on intrathecal drug delivery. J. Pain Res. 2014;7:615–626. doi: 10.2147/JPR.S37591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webster L.R., Fakata K.L., Charapata S., Fisher R., MineHart M. Open-label, multicenter study of combined intrathecal morphine and ziconotide: addition of morphine in patients receiving ziconotide for severe chronic pain. Pain Med. 2008;9:282–290. doi: 10.1111/j.1526-4637.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 54.Deer T.R., Pope J.E., Hanes M.C., McDowell G.C. Intrathecal therapy for chronic pain: a review of morphine and ziconotide as firstline options. Pain Med. 2019;20:784–798. doi: 10.1093/pm/pny132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deer T., Hagedorn J.M. How has ziconotide impacted non-cancer pain management? Expet Opin. Pharmacother. 2020;21:507–511. doi: 10.1080/14656566.2019.1707182. [DOI] [PubMed] [Google Scholar]
- 56.Wermeling D., Drass M., Ellis D., Mayo M., McGuire D., O'Connell D., Hale V., Chao S. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J. Clin. Pharmacol. 2003;43:624–636. [PubMed] [Google Scholar]
- 57.Schmidtko A.M., Lötsch J.P., Freynhagen R.M., Geisslinger G.P. Ziconotide for treatment of severe chronic pain. The Lancet (British edition) 2010;375:1569–1577. doi: 10.1016/S0140-6736(10)60354-6. [DOI] [PubMed] [Google Scholar]
- 58.Wallace M.S. Ziconotide: a new nonopioid intrathecal analgesic for the treatment of chronic pain. Expert Rev. Neurother. 2006;6:1423–1428. doi: 10.1586/14737175.6.10.1423. [DOI] [PubMed] [Google Scholar]
- 59.Lynch S.S., Cheng C.M., Yee J.L. Intrathecal ziconotide for refractory chronic pain. Ann. Pharmacother. 2006;40:1293–1300. doi: 10.1345/aph.1G584. [DOI] [PubMed] [Google Scholar]
- 60.Klotz U. Ziconotide--a novel neuron-specific calcium channel blocker for the intrathecal treatment of severe chronic pain--a short review. Int. J. Clin. Pharm. Ther. 2006;44:478–483. doi: 10.5414/cpp44478. [DOI] [PubMed] [Google Scholar]
- 61.Brookes M.E., Eldabe S., Batterham A. Ziconotide monotherapy: a systematic review of randomised controlled trials. Curr. Neuropharmacol. 2017;15:217–231. doi: 10.2174/1570159X14666160210142056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yaksh T.L., de Kater A., Dean R., Best B.M., Miljanich G.P. Pharmacokinetic analysis of ziconotide (SNX-111), an intrathecal N-type calcium channel blocking analgesic, delivered by bolus and infusion in the dog. Neuromodulation. 2012;15:508–519. doi: 10.1111/j.1525-1403.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko J.S., Eddinger K.A., Angert M., Chernov A.V., Dolkas J., Strongin A.Y., Yaksh T.L., Shubayev V.I. Spinal activity of interleukin 6 mediates myelin basic protein-induced allodynia. Brain Behav. Immun. 2016;56:378–389. doi: 10.1016/j.bbi.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deer T.R., Pope J.E., Hayek S.M., Bux A., Buchser E., Eldabe S., De Andres J.A., Erdek M., Patin D., Grider J.S., Doleys D.M., Jacobs M.S., Yaksh T.L., Poree L., Wallace M.S., Prager J., Rauck R., DeLeon O., Diwan S., Falowski S.M., Gazelka H.M., Kim P., Leong M., Levy R.M., McDowell G.I., McRoberts P., Naidu R., Narouze S., Perruchoud C., Rosen S.M., Rosenberg W.S., Saulino M., Staats P., Stearns L.J., Willis D., Krames E., Huntoon M., Mekhail N. The polyanalgesic consensus conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20:96–132. doi: 10.1111/ner.12538. [DOI] [PubMed] [Google Scholar]
- 65.Brogan S.E., Odell D.W., Sindt J.E., Yi I., Chrisman O.M., Zhang C., Presson A.P. Dorsal vs ventral intrathecal catheter tip location and effect on dose escalation and opioid use in patients with cancer pain. Neuromodulation. 2022;26:1233–1239. doi: 10.1016/j.neurom.2022.02.230. [DOI] [PubMed] [Google Scholar]
- 66.Vitale V., Battelli D., Gasperoni E., Monachese N. Intrathecal therapy with ziconotide: clinical experience and considerations on its use. Minerva Anestesiol. 2008;74:727–733. [PubMed] [Google Scholar]
- 67.Lin J.P., Zhang S.D., He F.F., Liu M.J., Ma X.X. The status of diagnosis and treatment to intracranial hypotension, including SIH. J. Headache Pain. 2017;18:4. doi: 10.1186/s10194-016-0708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bezov D., Lipton R.B., Ashina S. Post-dural puncture headache: part I diagnosis, epidemiology, etiology, and pathophysiology. Headache. 2010;50:1144–1152. doi: 10.1111/j.1526-4610.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- 69.Battal B., Kocaoglu M., Bulakbasi N., Husmen G., Tuba S.H., Tayfun C. Cerebrospinal fluid flow imaging by using phase-contrast MR technique. Br. J. Radiol. 2011;84:758–765. doi: 10.1259/bjr/66206791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holden R., Chauhan G., Emerick T. Intrathecal administration of ziconotide as a potential treatment for chronic migraines. Cureus J. Med. Sci. 2022;14 doi: 10.7759/cureus.23714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dowell D., Haegerich T.M., Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA, J. Am. Med. Assoc. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miljanich G.P. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004;11:3029–3040. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- 73.Staats P.S., Yearwood T., Charapata S.G., Presley R.W., Wallace M.S., Byas-Smith M., Fisher R., Bryce D.A., Mangieri E.A., Luther R.R., Mayo M., McGuire D., Ellis D. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA, J. Am. Med. Assoc. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- 74.Southwell D.G., Osorio J.A., Liverman C.S., Friedman L.M., Naidu R.K., Poree L.R., Henry M.M., Jacques L. Intrathecal catheter-associated inflammatory mass in a neurofibromatosis type-1 patient receiving fentanyl and bupivacaine. Surg. Neurol. Int. 2017;8:159. doi: 10.4103/sni.sni_80_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deer T.R., Malinowski M., Varshney V., Pope J. Choice of intrathecal drug in the treatment of neuropathic pain - new research and opinion. Expet Rev. Clin. Pharmacol. 2019;12:1003–1007. doi: 10.1080/17512433.2019.1659724. [DOI] [PubMed] [Google Scholar]
- 76.Wermeling D.P. Ziconotide, an intrathecally administered N-type calcium channel antagonist for the treatment of chronic pain. Pharmacotherapy. 2005;25:1084–1094. doi: 10.1592/phco.2005.25.8.1084. [DOI] [PubMed] [Google Scholar]
- 77.Ginalis E.E., Ali S., Mammis A. The role of intrathecal pumps in nonmalignant pain. Neurosurgery Clinics of America. 2022;33:305–309. doi: 10.1016/j.nec.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 78.Deer T.R., Hayek S.M., Pope J.E., Lamer T.J., Hamza M., Grider J.S., Rosen S.M., Narouze S., Perruchoud C., Thomson S., Russo M., Grigsby E., Doleys D.M., Jacobs M.S., Saulino M., Christo P., Kim P., Huntoon E.M., Krames E., Mekhail N. The polyanalgesic consensus conference (PACC): recommendations for trialing of intrathecal drug delivery infusion therapy. Neuromodulation: Technology at the Neural Interface. 2017;20:133–154. doi: 10.1111/ner.12543. [DOI] [PubMed] [Google Scholar]
- 79.Deer T.R., Pope J.E., Hayek S.M., Lamer T.J., Veizi I.E., Erdek M., Wallace M.S., Grider J.S., Levy R.M., Prager J., Rosen S.M., Saulino M., Yaksh T.L., De Andrés J.A., Abejon Gonzalez D., Vesper J., Schu S., Simpson B., Mekhail N. The polyanalgesic consensus conference (PACC): recommendations for intrathecal drug delivery: guidance for improving safety and mitigating risks. Neuromodulation: Technology at the Neural Interface. 2017;20:155–176. doi: 10.1111/ner.12579. [DOI] [PubMed] [Google Scholar]
- 80.Ridgeway B., Wallace M., Gerayli A. Ziconotide for the treatment of severe spasticity after spinal cord injury. Pain. 2000;85:287–289. doi: 10.1016/s0304-3959(99)00255-9. [DOI] [PubMed] [Google Scholar]
- 81.Stanton-Hicks M., Kapural L. An effective treatment of severe complex regional pain syndrome type 1 in a child using high doses of intrathecal ziconotide. J. Pain Symptom Manag. 2006;32:509–511. doi: 10.1016/j.jpainsymman.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Van Zundert J., Rauck R. Intrathecal drug delivery in the management of chronic pain. Best Pract. Res. Clin. Anaesthesiol. 2023;37:157–169. doi: 10.1016/j.bpa.2023.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Badve M., Shah T., Jones-Ivy S., Vallejo M.C. Ultrasound guided epidural analgesia for labor in a patient with an intrathecal baclofen pump. International Journal of Obstetric Anestjesia. 2011;20:370–372. doi: 10.1016/j.ijoa.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Adler J.A., Lotz N.M. Intrathecal pain management: a team-based approach. 2017;10:2565–2575. doi: 10.2147/JPR.S142147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ziconotide. 2006 PMID: 37247359. NBK592186. [Google Scholar]
- 86.Snutch T.P. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx. 2005;2:662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanford M. Intrathecal ziconotide: a review of its use in patients with chronic pain refractory to other systemic or intrathecal analgesics. CNS Drugs. 2013;27:989–1002. doi: 10.1007/s40263-013-0107-5. [DOI] [PubMed] [Google Scholar]
- 88.Wallace M.S., Charapata S.G., Fisher R., Byas-Smith M., Staats P.S., Mayo M., McGuire D., Ellis D. Intrathecal ziconotide in the treatment of chronic nonmalignant pain: a randomized, double-blind, placebo-controlled clinical trial. Neuromodulation. 2006;9:75–86. doi: 10.1111/j.1525-1403.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 89.Rauck R.L., Wallace M.S., Leong M.S., Minehart M., Webster L.R., Charapata S.G., Abraham J.E., Buffington D.E., Ellis D., Kartzinel R. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J. Pain Symptom Manag. 2006;31:393–406. doi: 10.1016/j.jpainsymman.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Deer T.R., Prager J., Levy R., Rathmell J., Buchser E., Burton A., Caraway D., Cousins M., De Andres J., Diwan S., Erdek M., Grigsby E., Huntoon M., Jacobs M.S., Kim P., Kumar K., Leong M., Liem L., McDowell G.N., Panchal S., Rauck R., Saulino M., Sitzman B.T., Staats P., Stanton-Hicks M., Stearns L., Wallace M., Willis K.D., Witt W., Yaksh T., Mekhail N. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436–464. doi: 10.1111/j.1525-1403.2012.00476.x. 464-466. [DOI] [PubMed] [Google Scholar]
- 91.Kress H.G., Simpson K.H., Marchettini P., Ver Donck A., Varrassi G. Intrathecal therapy: what has changed with the introduction of ziconotide. Pain Pract. 2009;9:338–347. doi: 10.1111/j.1533-2500.2009.00308.x. [DOI] [PubMed] [Google Scholar]
- 92.Low S.A., Robbins W., Tawfik V.L. Complex management of a patient with refractory primary erythromelalgia lacking a SCN9A mutation. J. Pain Res. 2017;10:973–977. doi: 10.2147/JPR.S129661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu X., Kohan L.R., Goldstein R.B. Low-dose intrathecal ziconotide for spasticity from primary lateral sclerosis: a case report. A & A Practice. 2019;13:31–33. doi: 10.1213/XAA.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 94.Lanzillo B., Loreto V., Calabrese C., Estraneo A., Moretta P., Trojano L. Does pain relief influence recovery of consciousness? A case report of a patient treated with ziconotide. Eur. J. Phys. Rehabil. Med. 2016;52:263–266. [PubMed] [Google Scholar]
- 95.Shao M.M., Khazen O., Hellman A., Czerwinski M., Dentinger R., DiMarzio M., Gillogly M., Hadanny A., Argoff C., Pilitsis J.G. Effect of first-line ziconotide intrathecal drug therapy for neuropathic pain on disability, emotional well-being, and pain catastrophizing. World Neurosurgery. 2021;145:e340–e347. doi: 10.1016/j.wneu.2020.10.079. [DOI] [PubMed] [Google Scholar]
- 96.Wallace M.S., Kosek P.S., Staats P., Fisher R., Schultz D.M., Leong M. Phase II, open-label, multicenter study of combined intrathecal morphine and ziconotide: addition of ziconotide in patients receiving intrathecal morphine for severe chronic pain. Pain Med. 2008;9:271–281. doi: 10.1111/j.1526-4637.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y.X., Gao D., Pettus M., Phillips C., Bowersox S.S. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive calcium channels, with morphine on nociception in rats. Pain. 2000;84:271–281. doi: 10.1016/s0304-3959(99)00214-6. [DOI] [PubMed] [Google Scholar]
- 98.Di Stefano V., Valdesi C., Zilli M., Peri M. Pancoast's syndrome caused by lymph node metastasis from breast cancer. BMJ Case Rep. 2018;11 doi: 10.1136/bcr-2018-226793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puntillo F., Giglio M., Preziosa A., Dalfino L., Bruno F., Brienza N., Varrassi G. Triple intrathecal combination therapy for end-stage cancer-related refractory pain: a prospective observational study with two-month follow-up. Pain and Therapy. 2020;9:783–792. doi: 10.1007/s40122-020-00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atanassoff P.G., Hartmannsgruber M.W., Thrasher J., Wermeling D., Longton W., Gaeta R., Singh T., Mayo M., McGuire D., Luther R.R. Ziconotide, a new N-type calcium channel blocker, administered intrathecally for acute postoperative pain. Reg. Anesth. Pain Med. 2000;25:274–278. doi: 10.1016/s1098-7339(00)90010-5. [DOI] [PubMed] [Google Scholar]
- 101.Hoederath P., Gautschi O.P., Land M., Hildebrandt G., Fournier J.Y. Formation of two consecutive intrathecal catheter tip granulomas within nine months. Cent. Eur. Neurosurg. 2010;71:39–42. doi: 10.1055/s-0029-1202359. [DOI] [PubMed] [Google Scholar]
- 102.McGivern J.G., McDonough S.I. Voltage-gated calcium channels as targets for the treatment of chronic pain. Curr. Drug Targets: CNS Neurol. Disord. 2004;3:457–478. doi: 10.2174/1568007043336743. [DOI] [PubMed] [Google Scholar]
- 103.Smith H.S., Deer T.R. Safety and efficacy of intrathecal ziconotide in the management of severe chronic pain. Therapeut. Clin. Risk Manag. 2009;5:521–534. doi: 10.2147/tcrm.s4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding X., Wang Y., Zhang S., Zhang R., Chen D., Chen L., Zhang Y., Luo S., Xu J., Pei C. Self-assembly nanostructure of myristoylated ω-conotoxin MVIIA increases the duration of efficacy and reduces side effects. Mar. Drugs. 2023;21:229. doi: 10.3390/md21040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olivera B.M., Teichert R.W. Diversity of the neurotoxic Conus peptides: a model for concerted pharmacological discovery. Mol. Interv. 2007;7:251–260. doi: 10.1124/mi.7.5.7. [DOI] [PubMed] [Google Scholar]
- 106.Newcomb R., Abbruscato T.J., Singh T., Nadasdi L., Davis T.P., Miljanich G. Bioavailability of Ziconotide in brain: influx from blood, stability, and diffusion. Peptides. 2000;21:491–501. doi: 10.1016/s0196-9781(00)00175-3. [DOI] [PubMed] [Google Scholar]
- 107.Ver Donck A., Vranken J.H., Puylaert M., Hayek S., Mekhail N., Van Zundert J. Intrathecal drug administration in chronic pain syndromes. Pain Pract. 2013;14:461–476. doi: 10.1111/papr.12111. [DOI] [PubMed] [Google Scholar]
- 108.Wilkes D. Programmable intrathecal pumps for the management of chronic pain: recommendations for improved efficiency. J. Pain Res. 2014;7:571–577. doi: 10.2147/JPR.S46929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Robert J., Sorrieul J., Rossignol E., Beaussart H., Kieffer H., Folliard C., Dupoiron D., Devys C. Chemical stability of morphine, ropivacaine, and ziconotide in combination for intrathecal analgesia. Int. J. Pharm. Compd. 2017;21:347–351. [PubMed] [Google Scholar]
- 110.Hsieh J.C., Penn R.D. Intrathecal baclofen in the treatment of adult spasticity. Neurosurg. Focus. 2006;21:e5. doi: 10.3171/foc.2006.21.2.6. [DOI] [PubMed] [Google Scholar]
- 111.Prager J., Jacobs M. Evaluation of patients for implantable pain modalities: medical and behavioral assessment. Clin. J. Pain. 2001;17:206–214. doi: 10.1097/00002508-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Prommer E.E. Ziconotide: can we use it in palliative care? Am. J. Hosp. Palliat. Med. 2005;22:369–374. doi: 10.1177/104990910502200510. [DOI] [PubMed] [Google Scholar]
- 113.Chung Y.H., Shin C., Park K.H., Cha C.I. Immunohistochemical study on the distribution of the voltage-gated calcium channel alpha(1B) subunit in the mature rat brain. Brain Res. 2000;866:274–280. doi: 10.1016/s0006-8993(00)02289-7. [DOI] [PubMed] [Google Scholar]
- 114.Mitchell A.A., Sapienza-Crawford A.J., Hanley K.L., Lokey K.J., Wells L. Using ziconotide for intrathecal infusions. Nurs. Res. Rev. 2008;38:19. doi: 10.1097/01.NURSE.0000342012.07225.e4. [DOI] [PubMed] [Google Scholar]
- 115.Webster L.R., Fisher R., Charapata S., Wallace M.S. Long-term intrathecal ziconotide for chronic pain: an open-label study. J. Pain Symptom Manag. 2009;37:363–372. doi: 10.1016/j.jpainsymman.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 116.Kisilevsky A.E., Mulligan S.J., Altier C., Iftinca M.C., Varela D., Tai C., Chen L., Hameed S., Hamid J., Macvicar B.A., Zamponi G.W. D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron. 2008;58:557–570. doi: 10.1016/j.neuron.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Löschner D., Dries R., Kalff R., Walter J., Reichart R. Was wurde eigentlich aus Prialt. Schmerz. 2021;35:343–348. doi: 10.1007/s00482-021-00531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang F., Yan Z., Liu Z., Wang S., Wu Q., Yu S., Ding J., Dai Q. Molecular basis of toxicity of N-type calcium channel inhibitor MVIIA. Neuropharmacology. 2016;101:137–145. doi: 10.1016/j.neuropharm.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 119.Schmidt-Rondon E., Wang Z., Malkmus S.A., Di Nardo A., Hildebrand K., Page L., Yaksh T.L. Effects of opioid and nonopioid analgesics on canine wheal formation and cultured human mast cell degranulation. Toxicol. Appl. Pharmacol. 2018;338:54–64. doi: 10.1016/j.taap.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sindt J.E., Larsen S.D., Dalley A.P., Collier W.H., Brogan S.E. The rate of infectious complications after intrathecal drug delivery system implant for cancer-related pain is low despite frequent concurrent anticancer treatment or leukopenia. Anesth. Analg. 2020;131:280–287. doi: 10.1213/ANE.0000000000004639. [DOI] [PubMed] [Google Scholar]
- 121.Atli A., Theodore B.R., Turk D.C., Loeser J.D. Intrathecal opioid therapy for chronic nonmalignant pain: a retrospective cohort study with 3-year follow-up. Pain Med. 2010;11:1010–1016. doi: 10.1111/j.1526-4637.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- 122.Teh H.S., Fadilah S.A., Leong C.F. Transverse myelopathy following intrathecal administration of chemotherapy. Singapore Medical Journal. 2007;48:e46–e49. [PubMed] [Google Scholar]
- 123.Huh B., Roldan C.J. Magnetic fields and intrathecal pump malfunction. AJEM (Am. J. Emerg. Med.) 2016;34:115. doi: 10.1016/j.ajem.2015.04.084. [DOI] [PubMed] [Google Scholar]
- 124.McDowell G.N., Winchell J. Role of primary care physicians in intrathecal pain management: a narrative review of the literature. PGM (Postgrad. Med.) 2018;130:411–419. doi: 10.1080/00325481.2018.1448207. [DOI] [PubMed] [Google Scholar]
- 125.Nair A.S., Poornachand A., Kodisharapu P.K. Ziconotide: indications, adverse effects, and limitations in managing refractory chronic pain. Indian J. Palliat. Care. 2018;24:118–119. doi: 10.4103/IJPC.IJPC_113_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nadherny W., Anderson B., Abd-Elsayed A. Perioperative and periprocedural care of patients with intrathecal pump therapy. Neuromodulation. 2019;22:775–780. doi: 10.1111/ner.12880. [DOI] [PubMed] [Google Scholar]
- 127.Brogan S.E., Sindt J.E., Odell D.W., Gulati A., Dupoiron D. Controversies in intrathecal drug delivery for cancer pain. Reg. Anesth. Pain Med. 2023;48:319–325. doi: 10.1136/rapm-2022-103770. [DOI] [PubMed] [Google Scholar]
- 128.Anand P., O'Neil A., Lin E., Douglas T., Holford M. Tailored delivery of analgesic ziconotide across a blood brain barrier model using viral nanocontainers. Sci. Rep. 2015;5 doi: 10.1038/srep12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Craik D.J., Fairlie D.P., Liras S., Price D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 130.Chow H.Y., Zhang Y., Matheson E., Li X. Ligation technologies for the Synthesis of cyclic peptides. Chem. Rev. 2019;119:9971–10001. doi: 10.1021/acs.chemrev.8b00657. [DOI] [PubMed] [Google Scholar]
- 131.Adebomi V., Cohen R.D., Wills R., Chavers H., Martin G.E., Raj M. CyClick chemistry for the Synthesis of cyclic peptides. Angewandte Chemie-international Edition. 2019;58:19073–19080. doi: 10.1002/anie.201911900. [DOI] [PubMed] [Google Scholar]
- 132.Dawson P.E., Muir T.W., Clark-Lewis I., Kent S.B. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 133.Song K., Hao Y., Tan X., Huang H., Wang L., Zheng W. Microneedle-mediated delivery of Ziconotide-loaded liposomes fused with exosomes for analgesia. J. Contr. Release. 2023;356:448–462. doi: 10.1016/j.jconrel.2023.03.007. [DOI] [PubMed] [Google Scholar]
- 134.Sanchez-Campos N., Bernaldez-Sarabia J., Licea-Navarro A.F. Conotoxin patenting trends in academia and industry. Mar. Drugs. 2022;20:531. doi: 10.3390/md20080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zamponi G.W., Lewis R.J., Todorovic S.M., Arneric S.P., Snutch T.P. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res. Rev. 2009;60:84–89. doi: 10.1016/j.brainresrev.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malve H. Exploring the ocean for new drug developments: marine pharmacology. J. Pharm. BioAllied Sci. 2016;8:83–91. doi: 10.4103/0975-7406.171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chakraborty K., Joy M. High-value compounds from the molluscs of marine and estuarine ecosystems as prospective functional food ingredients: an overview. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Winquist R.J., Pan J.Q., Gribkoff V.K. Use-dependent blockade of Cav2.2 voltage-gated calcium channels for neuropathic pain. Biochem. Pharmacol. 2005;70:489–499. doi: 10.1016/j.bcp.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 139.Feng Z.P., Doering C.J., Winkfein R.J., Beedle A.M., Spafford J.D., Zamponi G.W. Determinants of inhibition of transiently expressed voltage-gated calcium channels by omega-conotoxins GVIA and MVIIA. J. Biol. Chem. 2003;278:20171–20178. doi: 10.1074/jbc.M300581200. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Q., Ren Y., Mo Y., Guo P., Liao P., Luo Y., Mu J., Chen Z., Zhang Y., Li Y., Yang L., Liao D., Fu J., Shen J., Huang W., Xu X., Guo Y., Mei L., Zuo Y., Liu J., Yang H., Jiang R. Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects. Cell Res. 2022;32:461–476. doi: 10.1038/s41422-022-00616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Robert J., Sorrieul J., Dupoiron D., Jubier-Hamon S., Bienfait F., Visbecq A., Devys C. Physicochemical stability study of the morphine-ropivacaine-ziconotide association in implantable pumps for intrathecal administration. Neuromodulation: Technology at the Neural Interface. 2022;26:1179–1194. doi: 10.1016/j.neurom.2021.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.