Abstract
Objectives
The aim of this study was to evaluate variations in lateral ventricles in the examined feline population with the use of quantitative analysis methods to determine whether sex or body weight influenced the size of the ventricles, and to identify any significant differences in the results of low- and high-field MRI.
Methods
Twenty healthy European Shorthair cats, aged 1–3 years, with body weights ranging from 2.85–4.35 kg, were studied. MRI of brain structures was performed in a low- and a high-field MRI system. The height of the brain and lateral ventricles at the level of the interthalamic adhesion, and volume of the lateral ventricles were determined in T2-weighted images in the transverse plane. The degree of symmetry of lateral ventricles was analysed based on the ratio of right to left ventricular volume. The measured parameters were processed statistically to determine whether sex and body weight were significantly correlated with variations in ventricular anatomy. The results of low- and high-field MRI were analysed to evaluate for any significant differences.
Results
The average brain height was determined to be 27.79 mm, and the average height of the left and right ventricles were 2.98 mm and 2.89 mm, respectively. The average ventricle/brain height ratio was 10.61%. The average volume of the left ventricle was 134.12 mm3 and the right ventricle was 130.49 mm3. Moderately enlarged ventricles were observed in two cats. Moderate ventricular asymmetry was described in four cats. Sex and body weight had no significant effect on the evaluated parameters. The differences in the results of low- and high-field MRI were not statistically significant.
Conclusions and relevance
This study has determined reference intervals for ventricular volume in a population of European Shorthair cats without brain disease, which will facilitate the interpretation of MRI images and the characterisation of brain abnormalities in cats with neurological disease. Further research involving larger animal populations, including other breeds, is required to compare the measured parameters between breeds and to determine reference values for other breeds.
Introduction
MRI is a highly valuable and sensitive method for soft tissue analysis relying on high spatial resolution and tissue contrast. The resulting images are characterised by high quality with minimum artefacts.1–8
In veterinary medicine, MRI is used mainly to evaluate patients with suspected diseases of the central nervous system (CNS). 9 The cerebral ventricles in the brain are cavities filled with cerebrospinal fluid (CSF). The dilation of the ventricular system may occur as the result of a space-occupying lesion, or may be the result of obstruction of part of the ventricular system.5,8,10–12 Blockage of an interventricular foramen, third ventricle, mesencephalic aqueduct, fourth ventricle or lateral apertures may occur as the result of infection, neoplasia or haemorrhage.5,8 The dilation of ventricles may be also seen without any obvious cause of obstruction and presumed to be a congenital malformation. Marked or progressive ventriculomegaly is also described as hydrocephalus. Internal hydrocephalus is a group of disorders in which excessive CSF accumulates within the cerebral ventricles.5,8,9 External hydrocephalus describes an accumulation of CSF between the cerebral hemispheres and the overlying arachnoid membrane, rather than within the lateral ventricles. 13 In domestic animals, hydrocephalus frequently occurs secondary to obstruction of ventricular outflow of CSF caused by a pathological process such as infection or tumour, and is usually associated with changes in ventricular CSF pressure.8,9,11,14 However, in congenital forms of the disease, the accumulation of CSF is usually thought to represent an ex vacuo type of CSF accumulation caused by decreased formation or loss of brain tissue, which is generally not associated with changes in CSF flow and pressure.5,8–11
Any disease process causing ventriculomegaly with compression of the white matter adjacent to the ventricles can cause serious neurological impairments. 12 Recent studies demonstrated that lateral ventricular asymmetry and enlargement are relatively common in young and adult dogs without clinically apparent neurological signs.5,8 Furthermore, some canine breeds are predisposed to intrabreed variations in the anatomy of the cerebral ventricular system, including the Yorkshire Terrier, Beagle, German Shepherd and Labrador Retriever.15,16 Unfortunately, there is no analogous research related to cats. Variations in normal anatomy should be recognised when evaluating magnetic resonance (MR) scans of the animal brain.
The authors attempted to determine whether, similarly to selected dog breeds, the size and shape of the lateral cerebral ventricles varied considerably in a population of clinically normal European Shorthair cats. Attempts were also made to establish reference intervals for the analysed breed. The aim of this study was to evaluate the size and symmetry of lateral ventricles in one feline breed, to calibrate the gauge of ventricular height and volume, and to determine whether sex or body weight influence those parameters. The presence of statistically significant differences in the results of low- and high-field MRI was also determined.
Materials and methods
Twenty adult European Shorthair cats, including 10 males and 10 females, with a body weight of 2.85–4.35 kg (mean weight 3.54 kg), aged 1–3 years (mean age 2.3 years) were studied. The experimental animals were patients of a clinic operated by the Department of Surgery, and they were treated for reasons other than neurological disorders. As part of the diagnostic and research protocol, cats were handled in accordance with the Guidelines for the Care and Use of Laboratory Animals. The animals were subjected to a full physical and neurological examination. The cats were healthy and without clinical signs of CNS disorders. They were screened for metabolic diseases based on serum chemistry and complete blood count data. The cats were subjected to MRI in a low-field MR scanner with a 0.25 Tesla (T) magnet (Esaote Vet-MR Grande) and, 1 week later, in a high-field MRI scanner with a 3 T magnet (Siemens). The cats were subjected to general anaesthesia during both examinations. The procedures were carried out with the owners’ consent.
The cats were premedicated with atropine (Atropinum Sulfuricum; Polfa Warszawa) at 0.05 mg/kg body weight (BW) administered subcutaneously, medetomidine (Cepetor; ScanVet) at 0.1 mg/kg BW and midazolam (Midanium; Polfa Warszawa) at 0.1 mg/kg BW, administered by an intramuscular injection. General anaesthesia was induced with intravenous ketamine (Bioketan; Vetoquinol) at 2.5 mg/kg BW combined with propofol (Scanofol; ScanVet) at 1 mg/kg BW and maintained with the same combination of drugs administered intravenously to effect. The cats were intubated and oxygen was administered.
The animals were scanned. MR images were acquired with the use of a 0.25 T system. The cats were positioned in sternal recumbency with the head centred in the head coil. T2-weighted images of the brain were obtained in transverse, sagittal and dorsal planes. Transverse images were oriented in a position perpendicular to the hard palate. Dorsal and sagittal images were acquired perpendicular to the transverse plane. Dorsal and transverse images of the head were acquired by T2-weighted fast spin echo imaging with repetition time (TR) of 4010 ms and echo time (TE) of 90 ms. Sagittal T2-weighted images were acquired by fast spin echo imaging with TR of 4290 ms and TE of 120 ms. Slice thickness was 3 mm with no gap. In the high-field MRI system (3 T) images were acquired by placing the animals in sternal recumbency with the head centred inside the human wrist coil. T2-weighted images of the brain were obtained in identical planes in both systems. Slice thickness was 2 mm, with no gap.
T2-weighted images were analysed across the region of interest. The parameters of the brain and ventricles were quantified using computer image processing algorithms. Ventricular height (Vh) and brain height (Bh) were measured in transverse images at the level of the interthalamic adhesion. The ventricular area of the lateral ventricle was manually outlined in the transverse plane and calculated by a computer. Lateral ventricular volume (VV) was calculated as the sum of ventricular areas in each transverse image multiplied by slice thickness. The height and area of slit-like lateral ventricles were determined at 1 mm and 1 mm2, respectively. The ventricle to brain height ratio was calculated (Vh/Bh × 100%) for both lateral ventricles. The symmetry and size of lateral ventricles were assessed in view of the degree of ventricular asymmetry and enlargement based on previously published data for dogs.15,16 Lateral ventricles were classified as normal-sized (0–14% Vh/Bh), moderately enlarged (15–25% Vh/Bh) or severely enlarged (>25% Vh/Bh). The results were used to divide cats into groups with normal-sized lateral ventricles or unilateral/bilateral ventriculomegaly. The degree of asymmetry was determined based on the ratio between ventricular volumes (VV L/VV R) as normal (1 ± 0.03), minimal (1 ± 0.1), mild (1 ± 0.2) and severe (>1 ± 0.2).
The data were analysed using Statistica 12.5. Pearson’s correlation was used to examine the relationship between ventricular volume and body weight (H0 – there is no correlation between parameters for P value >0.05). Student’s t-test was used to examine the relationship between sex and ventricular volume (P values ⩽0.05 were considered as statistically significant). The data acquired at 0.25 T and 3 T were analysed with the Student’s paired t-test, in order to compare significant differences among Bh and Vh and volume. P values ⩽0.05 were considered to be statistically significant.
Results
All cats were successfully examined by low- and high-field MRI. The height of the feline brain at the level of the interthalamic adhesion was determined to be: 27.79 mm on average and with a range of 26.1–29.4 mm at 0.25 T; and 27.56 mm on average and with a range of 25.97–29.25 mm at 3 T. The average height of lateral ventricles measured at the same level at 0.25 T was 2.93 mm (2.98 mm for the left ventricle [range 1.8–4.2 mm] and 2.89 mm for the right ventricle [range 1.7–4.4 mm]), and at 3 T was 2.83 mm for the left ventricle (2.86 mm for the left ventricle [range 1.81–4.13 mm] and 2.81 mm for the right ventricle [range 1.55–4.21 mm]). The average ventricle/brain height ratio at 0.25 T was 10.61–10.77% for the right ventricle and 10.46% for the left ventricle. Moderate bilateral ventriculomegaly was noted in two cats, based on the ventricle/brain height ratio. One cat had unilateral ventricular enlargement on the left side when compared with the right, but both ventricular measurements were within the normal reference interval. Cerebral ventricles were of normal size in the remaining animals. Severe ventriculomegaly was not observed in any of the cats. The average ventricle/brain height ratio at 3 T was 10.34% (10.41% for the right ventricle and 10.26% for the left ventricle). The average combined left and right ventricular volume at 0.25 T was 264.61 mm3 (134.12 mm3 for the left ventricle [range 55.5–394.94 mm3] and 130.49 mm3 for the right ventricle [range 54–354.4 mm3]), and at 3T was 261.42 mm3 (132.24 mm3 for the left ventricle [range 53.14–387.65 mm3] and 129.18 mm3 for the right ventricle [range 53.89–350.72 mm3]). Ventricular volume exceeded the average more than three-fold in twocats that were diagnosed with moderate ventricular enlargement based on the ventricle/brain height ratio. In four cats, ventricular volume was two times lower than the average, and three of those animals weighed <3 kg. The relevant measurements are presented in Tables 1 and 2.
Table 1.
Ventricular measurements for all cats
| Cat | Sex | BW (kg) | 0.25 T | 3 T | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bh (mm) | Vh L (mm) | Vh R (mm) | Vh/Bh L (%) | Vh/Bh R (%) | VV L (mm3) | VV R (mm3) | Total VV | Left/right | Bh (mm) | Vh L (mm) | Vh R (mm) | Vh/Bh L (%) | Vh/Bh R (%) | VV L (mm3) | VV R (mm3) | Total VV | Left/right | |||
| 1 | M | 3.5 | 26.70 | 1.80 | 2.90 | 6.74 | 10.86 | 149.80 | 167.6 | 317.4 | 0.89 | 26.52 | 1.71 | 2.78 | 6.45 | 10.48 | 149.33 | 167.12 | 316.45 | 0.89 |
| 2 | F | 3 | 26.10 | 4.20 | 4.40 | 16.09 | 16.86 | 394.94 | 331.8 | 726.74 | 1.19 | 25.97 | 4.13 | 4.21 | 15.90 | 16.21 | 387.65 | 330.24 | 717.89 | 1.17 |
| 3 | F | 3.4 | 28.00 | 2.60 | 1.80 | 9.29 | 6.43 | 53.44 | 56.8 | 110.24 | 0.94 | 27.87 | 2.53 | 1.83 | 9.08 | 6.57 | 53.14 | 55.97 | 109.11 | 0.95 |
| 4 | M | 4.1 | 27.08 | 3.46 | 3.36 | 12.78 | 12.41 | 111.9 | 110.4 | 222.3 | 1.01 | 26.98 | 3.43 | 3.31 | 12.71 | 12.27 | 110.23 | 109.14 | 219.37 | 1.01 |
| 5 | F | 3.72 | 29.22 | 2.65 | 2.42 | 9.07 | 8.28 | 122.1 | 101.46 | 223.56 | 1.20 | 29.12 | 2.55 | 2.45 | 8.76 | 8.41 | 123.56 | 103.21 | 226.77 | 1.20 |
| 6 | M | 3.6 | 28.41 | 3.04 | 2.43 | 10.70 | 8.55 | 123.8 | 112.53 | 236.33 | 1.10 | 28.06 | 2.86 | 2.27 | 10.19 | 8.09 | 120.78 | 111.21 | 231.99 | 1.09 |
| 7 | F | 2.9 | 26.10 | 3.30 | 3.60 | 12.64 | 13.79 | 119.7 | 123.62 | 243.32 | 0.97 | 25.86 | 3.13 | 3.45 | 12.10 | 13.34 | 116.15 | 120.87 | 237.02 | 0.96 |
| 8 | M | 3.86 | 29.00 | 2.30 | 1.70 | 7.93 | 5.86 | 80.8 | 85.2 | 166 | 0.95 | 28.64 | 2.12 | 1.55 | 7.40 | 5.41 | 79.7 | 83.45 | 163.15 | 0.96 |
| 9 | F | 2.85 | 26.30 | 3.10 | 2.80 | 11.79 | 10.65 | 55.5 | 54 | 109.5 | 1.03 | 26.12 | 2.98 | 2.77 | 11.41 | 10.60 | 54.97 | 53.89 | 108.86 | 1.02 |
| 10 | F | 2.93 | 26.70 | 3.70 | 2.30 | 13.86 | 8.61 | 120 | 111.6 | 231.6 | 1.08 | 26.1 | 3.54 | 2.17 | 13.56 | 8.31 | 117.98 | 109.34 | 227.32 | 1.08 |
| 11 | M | 4.14 | 28.80 | 3.10 | 3.40 | 10.76 | 11.81 | 120.6 | 124.2 | 244.8 | 0.97 | 28.38 | 3.01 | 3.25 | 10.61 | 11.45 | 120.15 | 121.57 | 241.72 | 0.99 |
| 12 | M | 3.95 | 29.40 | 2.30 | 3.40 | 7.82 | 11.56 | 140.52 | 135.12 | 275.64 | 1.04 | 29.25 | 2.23 | 3.27 | 7.62 | 11.18 | 139.99 | 134.98 | 274.97 | 1.04 |
| 13 | F | 3.6 | 27.30 | 3.10 | 3.30 | 11.36 | 12.09 | 148.08 | 159.12 | 307.2 | 0.93 | 26.98 | 3.09 | 3.14 | 11.45 | 11.64 | 147.08 | 156.69 | 303.77 | 0.94 |
| 14 | F | 3.4 | 28.30 | 2.20 | 2.00 | 7.77 | 7.07 | 86.04 | 89.4 | 175.44 | 0.96 | 28.21 | 2.13 | 1.93 | 7.55 | 6.84 | 85.87 | 88.55 | 174.42 | 0.97 |
| 15 | F | 3.2 | 27.08 | 3.04 | 3.36 | 11.23 | 12.41 | 86.25 | 94.92 | 181.17 | 0.91 | 26.87 | 2.87 | 3.15 | 10.68 | 11.72 | 85.25 | 92.83 | 178.08 | 0.92 |
| 16 | M | 3.35 | 27.91 | 3.51 | 3.24 | 12.58 | 11.61 | 72.86 | 76.08 | 148.94 | 0.96 | 27.51 | 3.01 | 3.29 | 10.94 | 11.96 | 71.45 | 74.52 | 145.97 | 0.96 |
| 17 | M | 4.12 | 28.48 | 2.58 | 2.15 | 9.06 | 7.55 | 143.55 | 139.8 | 283.35 | 1.03 | 28.35 | 2.45 | 2.21 | 8.64 | 7.80 | 143.12 | 139.01 | 282.13 | 1.03 |
| 18 | F | 2.65 | 27.50 | 3.04 | 2.61 | 11.05 | 9.49 | 67.44 | 63 | 130.44 | 1.07 | 27.3 | 2.95 | 2.63 | 10.81 | 9.63 | 65.02 | 63.03 | 128.05 | 1.03 |
| 19 | M | 4.1 | 29.22 | 2.51 | 2.72 | 8.59 | 9.31 | 113.1 | 118.8 | 231.9 | 0.95 | 29.15 | 2.49 | 2.71 | 8.54 | 9.30 | 112.7 | 117.3 | 230 | 0.96 |
| 20 | M | 4.35 | 28.11 | 3.99 | 3.94 | 14.19 | 14.02 | 372 | 354.3 | 726.3 | 1.05 | 28.03 | 3.89 | 3.91 | 13.88 | 13.95 | 360.58 | 350.72 | 711.3 | 1.03 |
BW = body weight; Bh = brain height; Vh = ventricular height; Vh/Bh = ventricular to brain ratio; L = left; R = right; VV= ventricular volume; left/right = ratio of left to right ventricle; M = male; F = female
Table 2.
Comparison of quantitative data acquired at 0.25 T and 3 T
| 0.25 T | 3 T | |
|---|---|---|
| Mean Bh | 27.79 | 27.56 |
| Minimum Bh | 26.1 | 25.97 |
| Maximum Bh | 29.4 | 29.25 |
| Mean Vh L | 2.98 | 2.86 |
| Minimum Vh L | 1.8 | 1.71 |
| Maximum Vh L | 4.2 | 4.13 |
| Mean Vh R | 2.89 | 2.81 |
| Minimum Vh R | 1.7 | 1.55 |
| Maximum Vh R | 4.4 | 4.21 |
| Mean VV L | 134.12 | 132.24 |
| Minimum VV L | 55.5 | 53.14 |
| Maximum VV L | 394.94 | 387.65 |
| Mean VV R | 130.49 | 129.18 |
| Minimum VV R | 54 | 53.89 |
| Maximum VVR | 354.3 | 350.72 |
Bh = brain height; Vh = ventricular height; L = left; R = right; VV = ventricular volume
In four cases at 0.25 T and in seven cases at 3 T, the variations in lateral ventricular volume were <3% and classified as normal symmetry. Mild ventricular asymmetry was observed in four animals at 0.25 T and 3 T, and in three of those cases, the right ventricle was larger than the left ventricle. At 0.25 T minimal asymmetry was noted in 12 cases, where the right ventricle was larger in eight cats, and the left ventricle was larger in the remaining four. At 3 T minimal asymmetry was noted in nine cases, where the right ventricle was larger in seven cats, and the left ventricle was larger in the remaining two. The variations in ventricular volume and symmetry in the analysed animals are presented in Figures 1 and 2.
Figure 1.
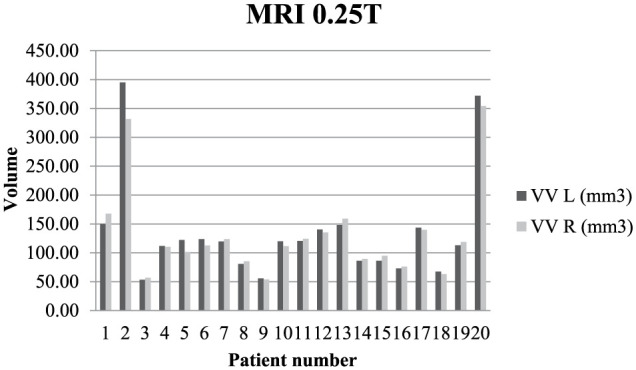
Variation in quantitative ventricular volume in 20 cats at 0.25 T. Note the mild asymmetry between the left and right ventricle in the first, second, fifth and sixth patients. Note the moderate ventriculomegaly in the second and 20th patients, and unilateral ventricular enlargement in fifth.
VV = ventricular volume; L = left; R = right
Figure 2.
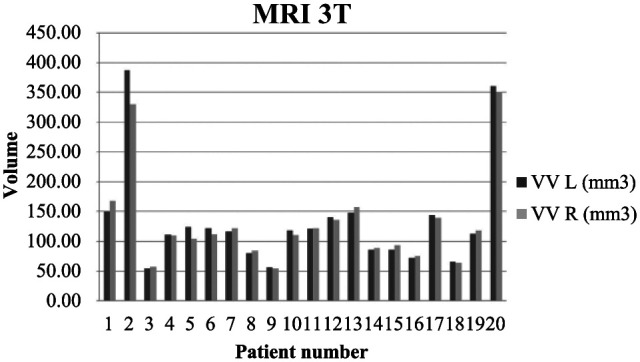
Variation in quantitative ventricular volume in 20 cats at 3 T. Note the mild asymmetry between the left and right ventricle in the first, fifth and sixth patients. Note the moderate ventriculomegaly and mild asymmetry in the second cat and moderate bilateral ventricular enlargement in the 20th cat.
VV = ventricular volume; L = left; R = right
There was no statistically significant correlation between ventricular size and body weight (P = 0.241; P >0.05). There was no statistically significant correlation between ventricular size and sex (P = 0.874; P >0.05). The statistical analysis of measured values of brain and ventricles at 0.25 T and 3 T were not significantly different, all P values <0.05 (Table 3).
Table 3.
Brain height, brains and height and volume of the left and right ventricles: mean values of data acquired at 0.25 T and 3 T
| 0.25 T | 3T | P value* | |||
|---|---|---|---|---|---|
| Height | Brain | 27.79 | 27.56 | 0.000001 | |
| Ventricle | Left | 2.98 | 2.86 | 0.000046 | |
| Right | 2.89 | 2.81 | 0.000814 | ||
| Volume | Ventricle | Left | 134.12 | 132.24 | 0.008279 |
| Right | 130.49 | 129.18 | 0.000110 |
P values of the Student’s t-test between 0.25 T and 3 T
Discussion
In the past, the quantification of cerebral ventricular volume was a complex process that relied solely on post-mortem examinations where ventricles were filled with water from a graduated cylinder or ventricular casts were measured in a fixed brain. Both methods had inherent technical difficulties and were unable to provide accurate measurements. For this reason, alterations in intracranial volume immediately before death may have increased intracranial pressure or induced cerebral oedema, and the calculated values were not necessarily consistent with in vivo measurements. Soft and unfixed brain tissue is difficult to handle, and cerebral volume shrinks with fixation.
Today, intracranial structures can be evaluated in vivo by CT or MRI, but these imaging methods are also associated with problems. 9 In MR images, the signals produced by CSF and oedema can have similar intensity. In canine hydrocephalus, cerebral oedema surrounding the ventricles is visualised in MRI. Therefore, ventricular volume can be calculated incorrectly when marginal oedema around cerebral ventricles is taken into consideration. Slice thickness can also contribute to error. Slices should be as thin as possible to guarantee that the volume of the ventricular system is calculated based on its shape and to prevent under- or overestimation of those values.
In this study, lateral ventricles were scanned by both low- and high-field MRI, and the results were compared to deliver the most precise measurements. The boundary between lateral ventricles and surrounding brain tissue is examined with greater precision in high-field MRI. High-field MRI is also characterised by higher spatial and contrast resolution, as well as the ability to scan 2 mm-thick slices, which lowers the risk of miscalculation. The differences in the measurements obtained by low- and high-field MRI were compared to determine the presence of statistical differences that would influence the interpretation of results.
The success of MRI relies on the choice of the appropriate examination protocol. 17 MRI sequences are designed by exploiting differences in the behaviour of hydrogen protons in various tissues exposed to a changing magnetic field. T1-weighted, T2-weighted andfluid-attenuated inversion recovery sequences are most commonly used for scanning brain tissues.4,18 T1-weighted images are considered to be more useful in evaluations of brain anatomy, while T2-weighted describes better pathology.16,19 Moreover, it has been reported that the ventricles of a healthy cat are too narrow to permit observations of ventricular walls. 20 In our research, we were able to observe ventricles in both T1- and T2-weighted images. According to spatial and contrast resolution, we observed better visibility of ventricular borders in high-field MRI. However, the accurate assessment of ventricular borders in T1-weighted images was harder than in T2-weighted images.
Malformations of the CNS in domestic animals mostly occur in association with gestational exposure to toxins or infectious agents, with death occurring at an early age. In dogs, congenital malformations of the CNS are common but often incidental findings, especially in toy breeds. 21 Moreover, enlargement and asymmetry of cerebral lateral ventricles are frequently noted in dogs, in particular in toy dog breeds, regardless of head shape.16,19 In cats, they are rare and associated occasionally with in utero parvoviral infections. 21 Ventriculomegaly is not always associated with clinical signs. The cerebral ventricular system has been investigated in various dog breeds to determine reference intervals indicative of normal ventricles, ventriculomegaly or hydrocephalus. The analysed breeds included the German Shepherd, Beagle, Yorkshire Terrier and English Bulldog, and the results revealed that reference intervals cannot be set for all breeds, owing to considerable interbreed variation. Differences were also observed within breeds, which further complicates the gauging process.16,19,22,23 Studies of this type have never been performed in cats and the reference intervals of feline lateral ventricles have never been published. Variation in the size of cerebral ventricles has not been studied previously within a feline breed or between breeds.
Recent research has demonstrated that the cerebral ventricular system can be accurately evaluated with three quantitative analysis methods. The ventricle/brain height was correlated with area and volume. One-, two- and three-dimensional quantitative methods are equally effective in evaluations of the ventricular system. 22 The imaging modality is generally selected based on a prior analysis of brain structure or brain parameters such as size and volume. Three complementary methods that rely on the height, area and volume of the brain and lateral ventricles have been used, to date, to produce statistically significant results. Ventricular volume is more important for evaluating the relative size and symmetry of ventricles and determining the shape of the entire cerebral ventricular system. 16 In a study of Beagles, Kii et al evaluated lateral ventricles based on the percentage of Vh to Bh. 17 Based on the resulting values, ventricles were classified as: normal-sized (0–14%), moderately enlarged (15–25%) and severely enlarged (>25%).
In this study, the same methodology was used to examine the feline ventricular system, which has not been extensively investigated to date. The aim of the experiment was to identify intrabreed differences in the size and symmetry of lateral ventricles, and to determine reference intervals for the European Shorthair. The results suggest that lateral ventricles are similar in size and fairly symmetrical in cats with similar BW. In the examined group, the average ventricular volume was exceeded three-fold in only two cats, including one that was also diagnosed with moderate ventricular asymmetry. No neurological disorders were observed in the examined animals. In four cases, ventricular volume was twice smaller than the average, and the ventricles were symmetrical or minimally asymmetrical. Three of these cats were characterized by below-average body weights and considerably smaller brain size, but normal proportions were maintained between brain size and ventricle size.
Conclusions
This study has determined reference intervals for ventricular volume in a population of European Shorthair cats without brain disease, which will facilitate the interpretation of MRI images and the characterisation of brain abnormalities in cats with neurological disease. Sex and body weight had no significant effect on the evaluated parameters. The differences in the results of low- and high-field MRI were not statistically significant. In our study the incidence of ventricular asymmetry was 20%, and this should be explored further in other breeds. Moreover, the results of this study offer valuable reference information for future research. Further work involving larger animal populations is needed to validate the present findings. In brachycephalic dog breeds, ventricular asymmetry and variations in ventricular size are relatively frequently noted, but are not often associated with clinical signs. A similar study should be undertaken in brachycephalic cats to examine differences in the anatomy of the feline ventricular system.
Acknowledgments
We would like to thank Professor Wojciech Maksymowicz for his support, and Miss Lucyna Nowak and Miss Kamila Milewska for their technical assistance during preparation of the manuscript.
Footnotes
Accepted: 23 September 2016
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kraft SL, Gavin PR, Wendling LR, et al. Canine brain anatomy on magnetic resonance images. Vet Radiol 1989; 30: 147–158. [Google Scholar]
- 2. Kang BT, Ko KJ, Jang DP, et al. Magnetic resonance imaging of the canine brain at 7T. Vet Radiol Ultrasound 2009; 50: 615–621. [DOI] [PubMed] [Google Scholar]
- 3. Gavin PR. Growth of clinical veterinary magnetic resonance imaging. Vet Radiol Ultrasound 2011; 52: S2–S4. [DOI] [PubMed] [Google Scholar]
- 4. Konar M, Lang J. Pros and cons of low-field magnetic resonance imaging in veterinary practice. Vet Radiol Ultrasound 2011; 52: S5–S14. [DOI] [PubMed] [Google Scholar]
- 5. MacKillop E. Magnetic resonance imaging of intracranial malformations in dogs and cats. Vet Radiol Ultrasound 2011; 52: S42–S51. [DOI] [PubMed] [Google Scholar]
- 6. Robertson I. Optimal magnetic resonance of the brain. Vet Radiol Ultrasound 2011; 52: S15–S22. [DOI] [PubMed] [Google Scholar]
- 7. Martin-Vaquero P, Da Costa RC, Echandi RL, et al. Magnetic resonance imaging of the canine brain at 3T and 7T. Vet Radiol Ultrasound 2011; 52: 25–32. [PubMed] [Google Scholar]
- 8. Przyborowska P, Adamiak Z, Jaskolska M, et al. Hydrocephalus in dogs: a review. Vet Med (Praha) 2013; 58: 73–80. [Google Scholar]
- 9. Tani K, Taga A, Itamoto K, et al. Hydrocephalus and syringomyelia in a cat. J Vet Med Sci 2001; 63: 1331–1334. [DOI] [PubMed] [Google Scholar]
- 10. Fenghong L, Scott L, Songbai J, et al. Model-based estimation of ventricular deformation in the cat brain. Med Image Comput Comput Assist Interv 2009; 12: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klaric M, Oreskovic D, Bozic B, et al. New experimental model of acute aqueductal blockage in cats: effects on cerebrospinal fluid pressure and the size of brain ventricles. Neuroscience 2009; 158: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 12. Okada M, Kitagawa M, Ito D, et al. MRI of secondary cervical syringomyelia in four cats. J Vet Med Sci 2009; 71: 1069–1073. [DOI] [PubMed] [Google Scholar]
- 13. Dewey CW, Coates JR, Ducoté JM, et al. External hydrocephalus in two cats. J Am Anim Hosp Assoc 2003; 39: 567–572. [DOI] [PubMed] [Google Scholar]
- 14. Pattison AJ, Lollis SS, Perriñez PR, et al. Time-harmonic magnetic resonance elastography of the normal feline brain. J Biomech 2010; 19: 2747–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vullo T, Korenman E, Manzo RP, et al. Diagnosis of cerebral ventriculomegaly in normal adult beagles using quantitative MRI. Vet Radiol Ultrasound 1997; 38: 277–281. [DOI] [PubMed] [Google Scholar]
- 16. Kii S, Uzuka Y, Taura Y, et al. Magnetic resonance imaging of the lateral ventricles in beagle-type dogs. Vet Radiol Ultrasound 1997; 38: 430–433. [DOI] [PubMed] [Google Scholar]
- 17. Jaskolska M, Adamiak Z, Zhalniarovich Y, et al. Magnetic resonance imaging protocols in equine lameness examination, useds equences, and interpretation. Pol J Vet Sci 2013; 4: 803–811. [DOI] [PubMed] [Google Scholar]
- 18. Hecht S, Adams WH. MRI of brain disease in veterinary patients part 1: basic principles and congenital brain disorders. Vet Clin North Am Small Anim Pract 2010; 40: 21–38. [DOI] [PubMed] [Google Scholar]
- 19. Esteve-Ratsch B, Kneissl S, Gabler C. Comparative evaluation of the ventricles in the Yorkshire Terrier and the German Shepherd dog using low-field MRI. Vet Radiol Ultrasound 2001; 42: 410–413. [DOI] [PubMed] [Google Scholar]
- 20. Hudson LC, Cauzinille L, Kornegay JN, et al. Magnetic resonance imaging of the normal feline brain. Vet Radiol Ultrasound 1995; 36: 267–275. [Google Scholar]
- 21. Keating MK, Sturges BK, Sisó S, et al. Characterization of an inherited neurologic syndrome in Toyger cats with forebrain commissural malformations, ventriculomegaly and interhemispheric cysts. J Vet Intern Med 2016; 30: 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woo DC, Choi CB, Nam JW, et al. Quantitative analysis of hydrocephalic ventricular alterations in Yorkshire terriers using magnetic resonance imaging. Vet Med (Praha) 2010; 55: 125–132. [Google Scholar]
- 23. Vite CH, McGowan JC, Niogi SN, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol 2005; 57: 355–364. [DOI] [PubMed] [Google Scholar]


