Fig. 1: Circulating host-derived EVs capture LPS.
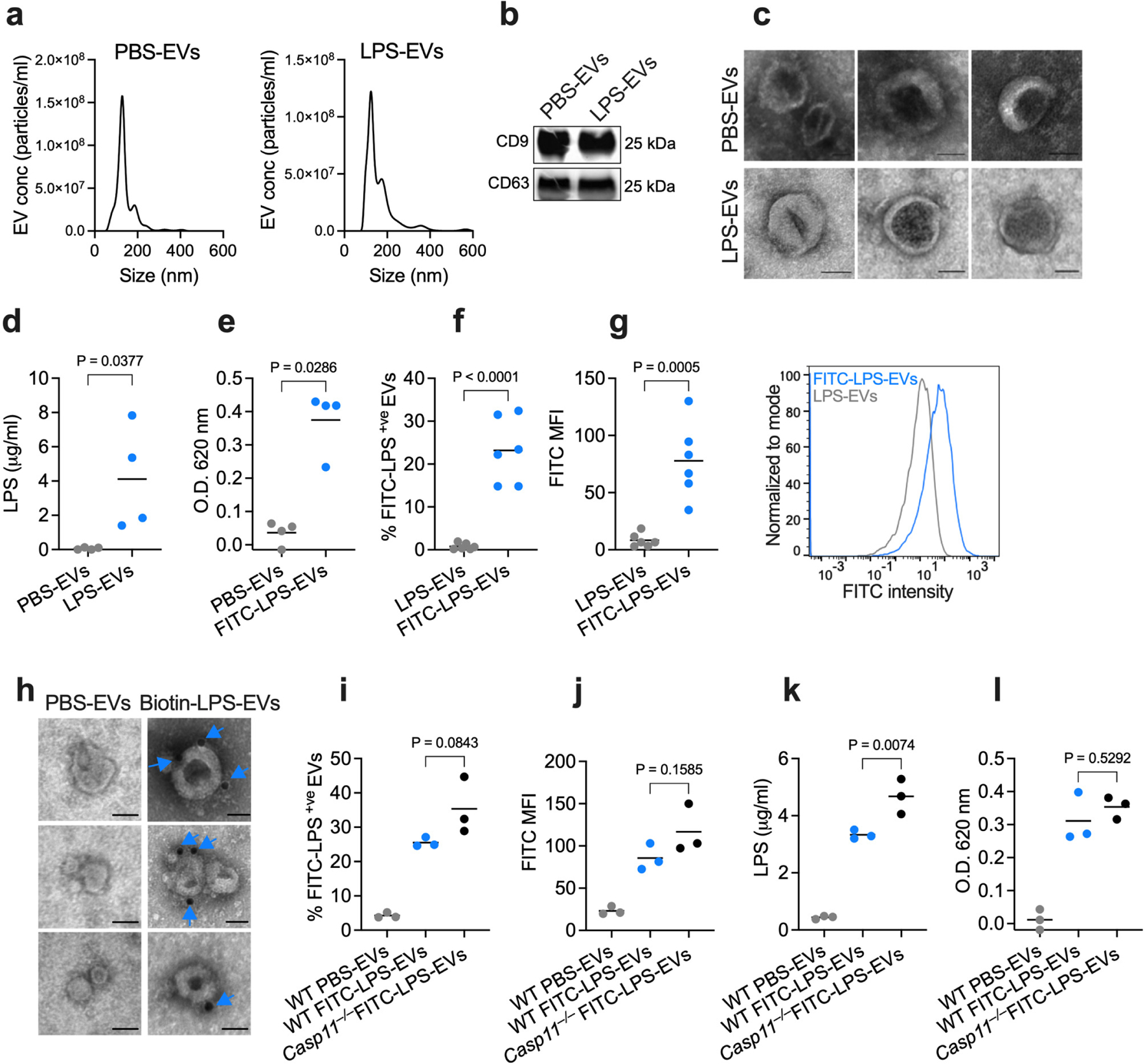
a, Nanoparticle tracking analysis (NTA) of the size distribution of EVs isolated from the plasma of wild-type (WT) mice injected with PBS or LPS (25 mg/kg) 1.5 h post-injection via ultracentrifugation. b, Immunoblotting analysis of EVs isolated from the plasma of PBS- or LPS-injected WT mice for CD9 and CD63. c, Negative staining transmission electron microscopy (TEM) of EVs isolated from the plasma of PBS- or LPS-injected WT mice as in (a). d,e, LPS content of the EVs isolated from WT mice injected with PBS, LPS or FITC-LPS (25 mg/kg) 1.5 h post-injection via ultracentrifugation as assessed by the LAL (n=4) (d) and HEK-Blue TLR4 reporter cell (e) assays (n=4). f,g, Percentage of FITC-LPS+ve EVs (f) and FITC histogram and mean fluorescence intensity (MFI) of EVs (g) isolated from mice injected with unlabeled LPS or FITC-LPS 1.5 h post-injection via ultracentrifugation as assessed by ImageStream flow cytometry (n=6). h, TEM of EVs isolated from the plasma of mice injected with PBS- or biotin-LPS 1.5 h post-injection and stained with streptavidin-gold particles. Arrows indicate LPS. i,j, Percentage of FITC-LPS+ve EVs (i) and FITC MFI of EVs (j) isolated from WT and Casp11−/− mice injected with PBS or FITC-LPS 1.5 h post-injection via ultracentrifugation as assessed by ImageStream flow cytometry (n=3). k,l, LPS content of the EVs isolated from WT and Casp11−/− mice injected with PBS or FITC-LPS 1.5 h post-injection via ultracentrifugation as assessed by the LAL (k) and HEK-Blue TLR4 reporter cell (l) assays (n=3). Combined data from three (i–l) four (d,e) or six (f,g) independent experiments or data from one experiment of representative of two (b,c and h) or three (a) are shown. Each circle represents a mouse, and the horizontal lines represent the mean (d–g,i–l). P values were determined by unpaired two-tailed t-test (d–g), one-way ANOVA with Dunnett’s post-test (i–l). Scale bar, 50 nm (c,h).
