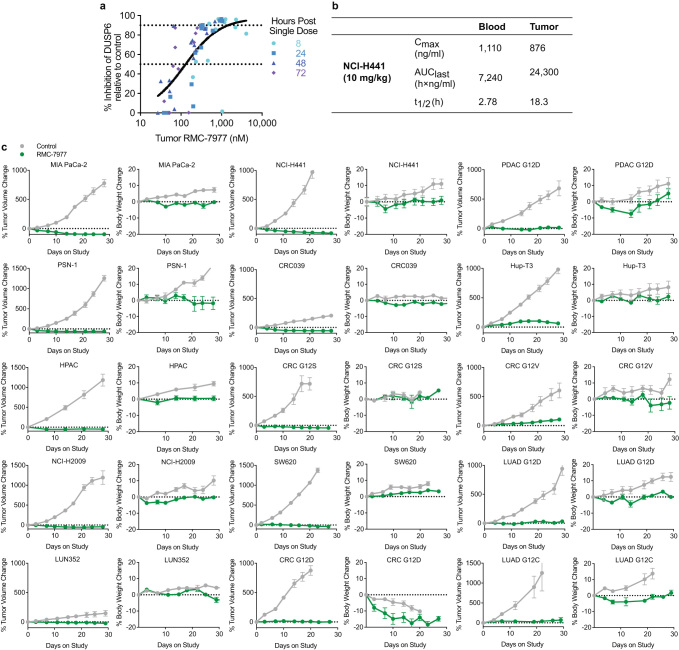
Extended Data Fig. 8. RMC-7977 PKPD, tumour volumes, and body weights of tumour bearing mice.
a, Pharmacokinetic (PK) relationship to pharmacodynamic (PD) marker (DUSP6) levels. RMC-7977 tumour concentrations and percentage of DUSP6 inhibition in tumour following single oral administration in NCI-H441 (KRASG12V, NSCLC) tumour bearing BALB/c nude mice. All datapoints shown, n = 3 per dose and timepoint. EC50 = 130 nM and an EC90 = 1,450 nM depicted by horizontal dotted lines, with a maximal level of inhibition near 100% relative to control. b, Table of blood and tumour PK parameters of RMC-7977 following single oral administration of RMC-7977 in NCI-H441 tumour bearing BALB/c nude mice. Whole blood and tumour concentrations of RMC-7977 were determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS). PK parameters were calculated by non-compartmental analysis (Phoenix WinNonlin). c, Percent tumour volume change and percent body weight change of tumour bearing BALB/c nude mice following repeated oral administration of either vehicle control or RMC-7977 at 10 mg/kg. Treatments were administered daily for 7 days per week except for CRC G12V, LUAD G12D, CRC G12S, LUAD G12C, and PDAC G12D, which were all treated once daily for 5 days of treatment followed by 2 days of treatment cessation every week. Tolerability defined as body weight loss <20%. n = 3–28 mice per group, datapoints represent mean ±s.e.m. normalized to initial (day 0, depicted as a horizontal dotted line) measurements.