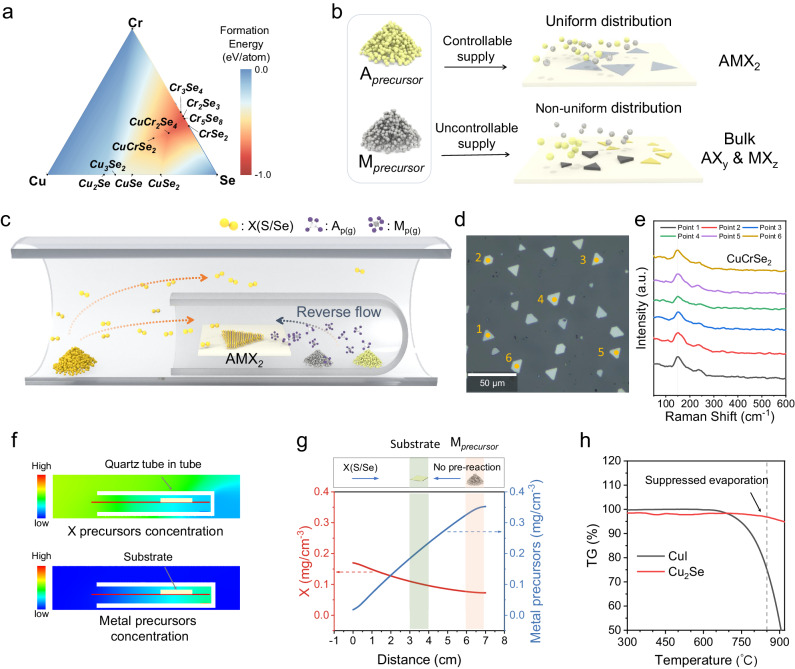
Fig. 1. Synthesis mechanism of chemical vapor deposition (CVD) growth AMX2.
a The formation energy of CuCrSe2 phase diagram35. b The kinetic growth process is influenced by the supply of metal precursors. A and M present the two kinds of metal elements of AMX2 compounds, and X represents the chalcogen element. The x and y in the AXy and MXz demonstrate the possible stoichiometric ratio of the binary by-products in the (a) (such as CuSe, Cu2Se, etc.). c Schematic image of the CVD setup. The orange and blue dash arrows represent the transportation paths of the vapored chalcogen precursor and metal precursors (Ap(g) and Mp(g)), respectively. d The large area optical image of the as-synthesized CuCrSe2 nanosheets. e The Raman spectra of the flakes in the (d). The vertical dash line located at 150 cm−1 demonstrates the consistent Raman peaks of the as-synthesized CuCrSe2 nanosheets. f The computational fluid dynamics (CFD) simulated distribution of X (S/Se) and metal precursors concentration. g The CFD simulated variation curve of precursor concentration along the red line in the (f). The green and pink shaded areas schematically represent the position of the substrate and metal precursors, respectively. h Thermogravimetric analysis (TGA) of CuI and Cu2Se powders. The black and red curves correspond to the weight-loss curves of CuI and Cu2Se, respectively. The vertical dash line located at 850°C demonstrates that the evaporation of excessively selenated metal precursors will be significantly suppressed.