Figure 3. Immuno-Electron Microscopy Ultrastructural Localization of Cav1.2 Channels, SK3 Channels, and β1-Adrenoceptor (β1-AR) in Layer III of the Dorsolateral Prefrontal Cortex (dlPFC) in Macaques.
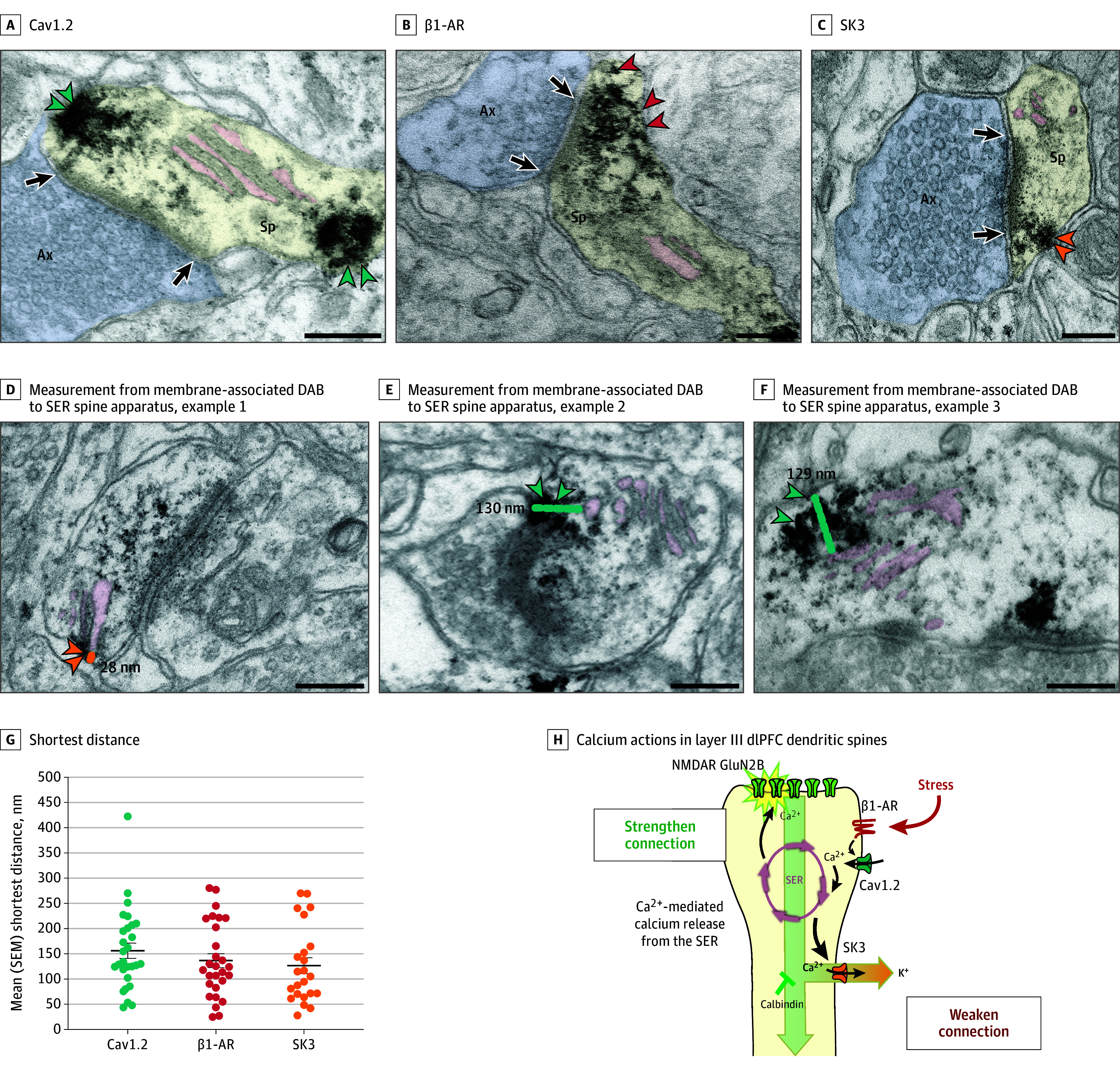
Cav1.2 (A, teal arrowheads), β1-AR (B, red arrowheads), and SK3 channels (C, orange arrowheadss) can be seen in dendritic spines receiving asymmetric (presumed glutamatergic) synapses, often localized on the plasma membrane near the calcium-storing and -releasing smooth endoplasmic reticulum (SER; termed the spine apparatus when it is elaborated in the dendritic spine). The SER spine apparatus is highlighted with pink pseudocoloring. Note that the Cav1.2 labeling can be seen near the postsynaptic density (PSD) and near the SER; additional examples can be seen in eFigures 3 and 4 in Supplement 1. Calbindin was not examined, as it is a cytosolic protein with diffuse labeling. D-F, Examples of measurements from center of membrane-associated diaminobenzidine (DAB) label to the SER spine apparatus. G, Shortest distance measured from center of membrane-associated DAB Cav1.2 (teal), β1-AR (red), and SK3 (orange) channel label to the SER spine apparatus; each dot represents a spine, and the black bar depicts the mean, with error bars indicating SEMs. Note that the DAB label may have obscured the SER spine apparatus in some instances; thus, the proteins on the plasma membrane may have been even closer than measured. H, A working model of calcium actions in layer III dlPFC dendritic spines, showing a functional calcium-related interactome. Under nonstress conditions, moderate Cav1.2 L-type voltage-gated Ca2+ channel actions, including potential calcium-mediated calcium release through ryanodine receptors (RYR) on the SER spine apparatus, are needed for strong working memory delay-related firing, possibly by depolarizing the postsynaptic density (PSD) to permit NMDA receptor (NMDAR) neurotransmission. Previous research has shown that the PSD contains NMDA receptors with GluN2B subunits, and that delay cell firing during working memory depends on NMDA receptor–GluN2B neurotransmission. Under stressful conditions, high levels of norepinephrine stimulate β1-AR to activate a large number of L-type calcium channel (LTCC)/Cav1.2 calcium channels. This may induce high levels of calcium-mediated calcium release from the SER, as occurs with the stress response in the heart. High levels of calcium would open large numbers of SK potassium (K+) channels, rapidly reducing neuronal firing. Feedforward, calcium–cyclic adenosine monophosphate signaling would also open cyclic adenosine monophosphate–sensitive channels on spines (eg, hyperpolarization-activated and cyclic nucleotide–gated slack [K+]) channels. As dlPFC delay cell firing is needed for working memory, these intracellular signaling events lead to cognitive impairment. Sustained reductions in neuronal firing or high levels of cytosolic calcium would also lead to degeneration, especially when the protective effects of calbindin are lost with age and/or inflammation. Black arrows indicate synapses. Ax indicates axon terminal (pseudocolored blue); Mit, mitochondria; Sp, spine (pseudocolored yellow). Scale bars = 200 nm.
