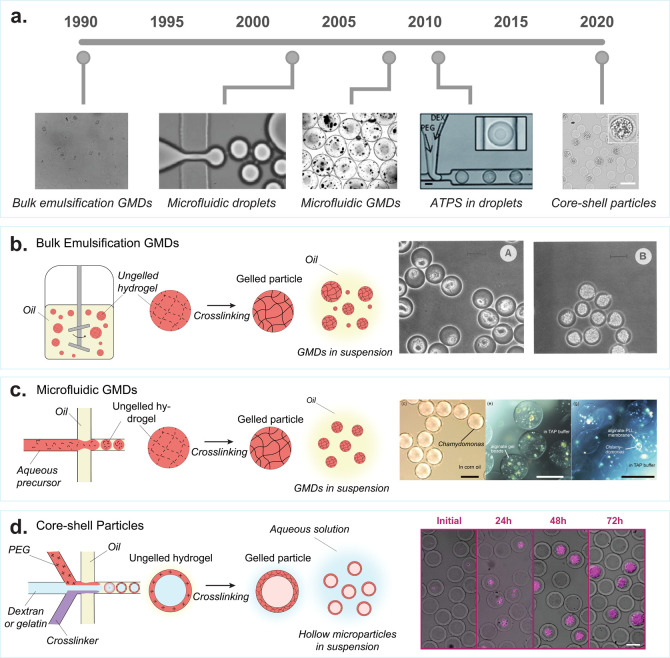
Figure 2.
Evolution of cell encapsulation in hydrogel drops for single-cell analysis. (a) Some of the first hydrogel microparticles for cell and protein screening were made using bulk emulsification methods, producing polydisperse agarose particles called gel microdrops (GMDs). [Reprinted with permission from Macmillan Publishers Ltd.: Nature, Weaver, J. C., et al. Nat Biotechnol1988, 6 (9), 1084–1089 (ref (41)) Copyright 1988.] Droplet microfluidics became popular in the early 2000s [Reprinted with permission from ref (42). Anna, S. L., et al. Applied Physics Letters2003, 82 (3), 364–366, 2003 licensed under a Creative Common Attribution (CC BY) license], which was later used to encapsulate cells in uniform GMDs [Reproduced from On-Chip Alginate Microencapsulation of Functional Cells Workman, V, et al. Macromol. Rapid Commun.2008, 29 (2), 165–170 (ref (43)) Copyright 2008 Wiley]. The first studies of aqueous two-phase systems in microfluidic droplets started in the early 2010s. Scale bar is 50 μm. [Reproduced from Vijayakumar, K., et al. Chemical Science2010, 1 (4), 447–452 (ref (44)) with permission from The Royal Society of Chemistry.] This led to the development of hollow hydrogel microparticles that form around encapsulated cells. Scale bar is 50 μm. [Reproduced from Leonaviciene, G., et al. Lab Chip2020, 20 (21), 4052–4062 (ref (45)) with permission from The Royal Society of Chemistry.] (b) Bulk emulsion GMDs: Formation of polydisperse GMDs by vigorously mixing an ungelled polymer solution in oil, to create water-in-oil emulsions. These emulsions, once stabilized, are gelled to form nonuniform GMDs. The oil is removed, and the GMDs are transferred to an aqueous solution. Scale bar is 20 μm. [Reprinted with permission from ref (46). Copyright, 1990 American Society for Microbiology.] (c) Microfluidic GMDs: A microfluidic droplet generator is used to create monodisperse water-in-oil droplet emulsions consisting of ungelled polymer precursors. Subsequently, the solution undergoes gelation to form uniform GMDs. After gelation, the oil is removed, and the GMDs are transferred into an aqueous solution. Scale bar is 100 μm. [Reproduced from Morimoto, Y., et al. Lab Chip2009, 9 (15), 2217–2223 (ref (47)) with permission from The Royal Society of Chemistry.] (d) Core–shell particles: An aqueous two-phase system is employed within a microfluidic droplet generator to produce core–shell microparticles featuring a hollow inner cavity. Polyethylene glycol (PEG), dextran, and a cross-linker are combined to form a water-in-oil emulsion using the microfluidic droplet generator. PEG and dextran undergo phase separation; dextran moves toward the center, while PEG aligns at the surface of the emulsion. Subsequently, the PEG-rich phase is cross-linked to create a solid outer shell. Afterward, the particle is transitioned from oil to water. During this transfer, the inert dextran escapes through the pores of the outer shell, resulting in a hollow interior. Scale bar is 50 μm. [Reproduced with permission from Proceedings of the National Academy of Sciences USA van Zee, M, et al. Proc. Natl. Acad. Sci. U.S.A.2022, 119 (4), e2109430119 (ref (48)).]